
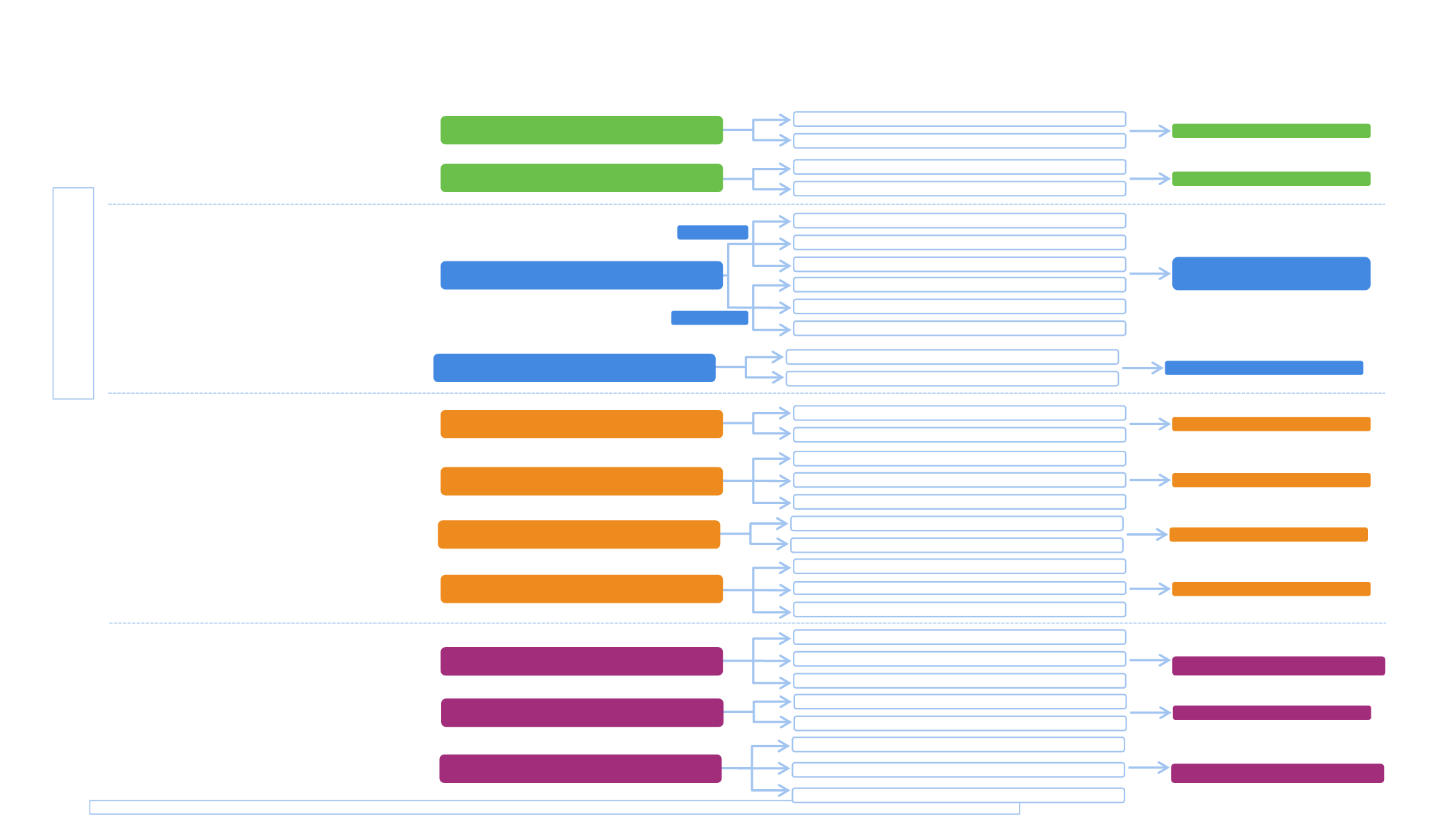
Selected phase 3 combination studies with immune checkpoint inhibitors in 1
st
-line
advanced NSCLC
Pembrolizumab
Durvalumab
SOC=standard of care. ClinicalTrials.gov.
http://www.clinicaltrials.gov/.Accessed September 2017.
Atezolizumab
Anti-PD-1/PD-L1
KEYNOTE-407
Pembrolizumab+Carbo+Paclitaxel (or nab)
Placebo+Carbo+Paclitaxel (or nab)
Primary endpoint: OS, PFS
Stage IV Squamous NSCLC
N=560
Nivolumab
Primary endpoints:
OS, PFS
CheckMate 227
Part 1
Nivolumab + ipilimumab
Nivolumab
Platinum-based chemotherapy
Stage IV o recurrent NSCLC
N=2220
KEYNOTE-189
Pembrolizumab+Platinum+ Pemetrexed
Placebo+Platinum+ Pemetrexed
Primary endpoint: PFS
Stage IV Non squamous NSCLC
N=570
IMpower 150
Atezolizumab + carboplatin + paclitaxel
Bevacizumab + paclitaxel + carboplatin
Primary endpoint: PFS, OS
Atezolizumab + bevacizumab + paclitaxel + carboplatin
Stage IV non-squamous NSCLC
N=1202
IMpower 130
Atezolizumab + carboplatin + nab-paclitaxel
Carboplatin + nab-paclitaxel
Primary endpoint: PFS, OS
Stage IV non-squamous NSCLC
N=724
IMpower 131
Atezolizumab + carboplatin + nab-paclitaxel
Carboplatin + nab-paclitaxel
Primary endpoint: PFS, OS
Atezolizumab + carboplatin + paclitaxel
Stage IV squamous NSCLC
N=1021
Primary endpoint: PFS, OS
MYSTIC
Durvalumab
Durvalumab + tremelimumab
SOC chemotherapy
Stage IV NSCLC
N=1118
Nivolumab + ipilimumab
Nivolumab + chemotherapy
Platinum-based chemotherapy
IMpower 132
Atezolizumab + platinum+ pemetrexed
Platinum + pemetrexed
Primary endpoint: PFS, OS
Stage IV non-squamous NSCLC
N=568
NEPTUNE
Durvalumab + tremelimumabl
SOC chemotherapy
Primary endpoint: OS
Stage IV NSCLC
N=960
Primary endpoint: PFS
POSEIDON
Durvalumab + SOC chemotherapy
Durvalumab + tremelimumab + SOC chemotherapy
SOC chemotherapy
Stage IV NSCLC
N=801
CheckMate 227
Part 2
Nivolumab + chemotherapy
Chemotherapy
Primary endpoint: PFS, OS
Stage IV or recurrent NSCLC
N=480
PD-L1≥1%
PD-L1<1%









