
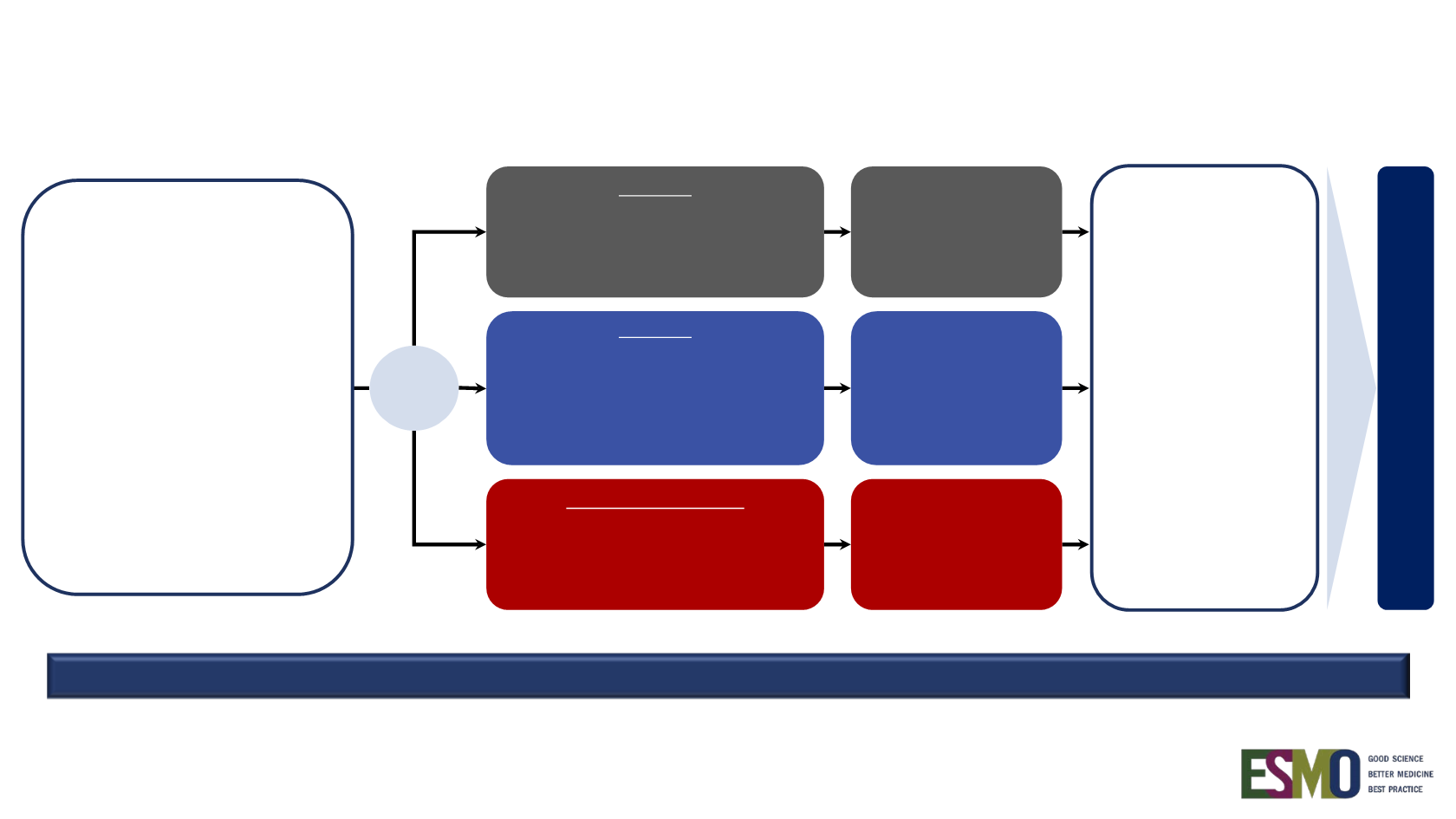
Reck M, et al. IMpower150 PFS analysis.
32
a
Patients with a sensitising
EGFR
mutation or
ALK
translocation must have disease progression or intolerance of treatment with
one or more approved targeted therapies.
b
Atezolizumab: 1200 mg IV q3w.
c
Carboplatin: AUC 6 IV q3w.
d
Paclitaxel: 200 mg/m
2
IV q3w.
e
Bevacizumab: 15 mg/kg IV q3w.
IMpower150 study design
Arm A
Atezolizumab
b
+
Carboplatin
c
+ Paclitaxel
d
4 or 6 cycles
Atezolizumab
b
Arm C
(control)
Carboplatin
c
+ Paclitaxel
d
+
Bevacizumab
e
4 or 6 cycles
Bevacizumab
e
Survival follow-up
Stage IV or
recurrent metastatic
non-squamous NSCLC
Chemotherapy-naive
a
Tumour tissue available
for biomarker testing
Any PD-L1 IHC status
Stratification factors:
• Sex
• PD-L1 IHC expression
• Liver metastases
N = 1202
R
1:1:1
Arm B
Atezolizumab
b
+
Carboplatin
c
+ Paclitaxel
d
+
Bevacizumab
e
4 or 6 cycles
Atezolizumab
b
+
Bevacizumab
e
Maintenance therapy
(no crossover permitted)
Treated with
atezolizumab
until PD by
RECIST v1.1
or loss of
clinical benefit
AND/OR
Treated with
bevacizumab
until PD by
RECIST v1.1
The principal question is to assess whether the addition of atezolizumab to Arm C provides clinical benefit









