
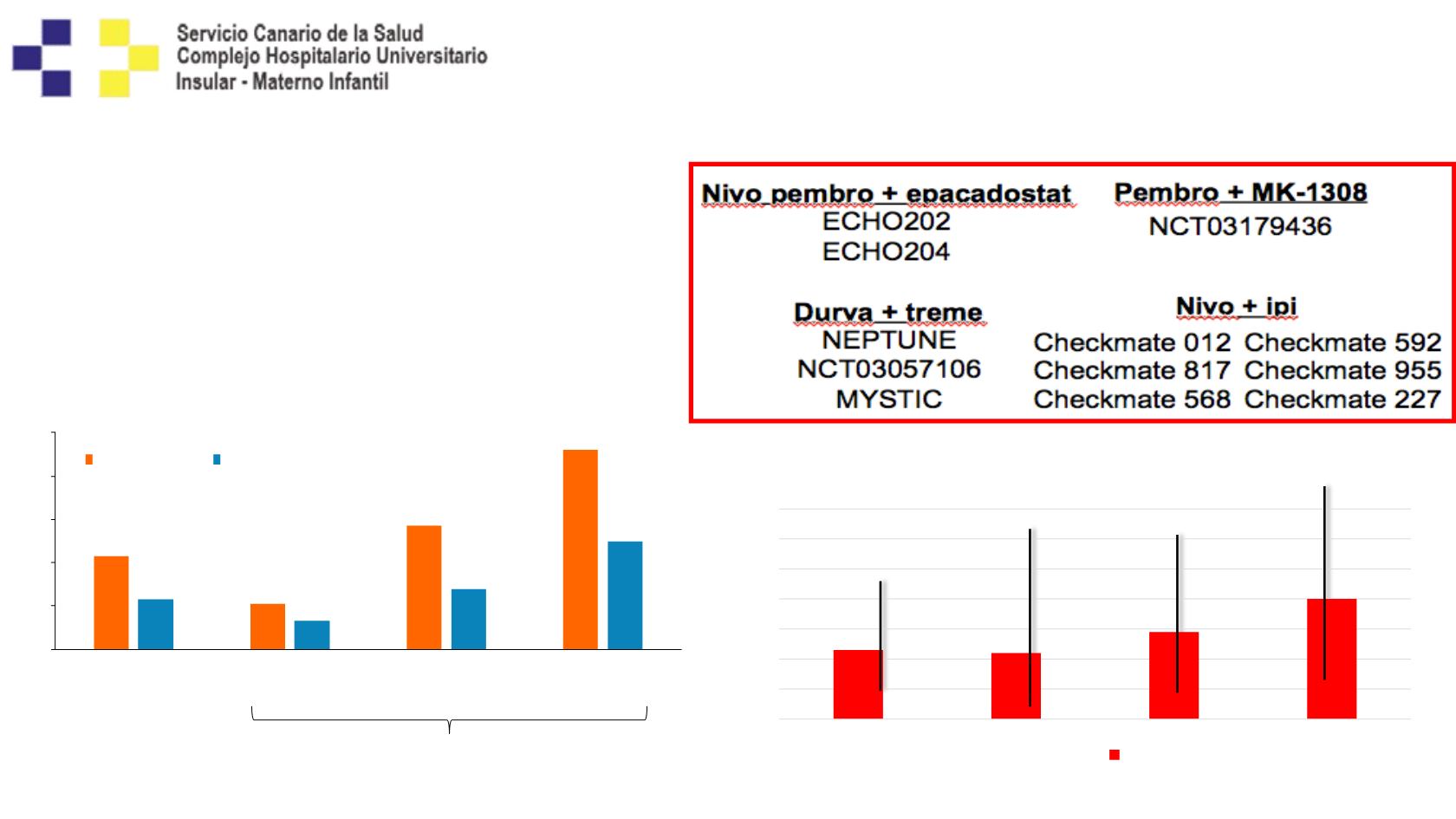
IO-IO Combinations (Phase I)
(Nivolumab/Ipilimumab – Durvalumab/Tremelimumab)
• Response: 23-43%
• Substantial Toxicity
• Conflicting Impact of PD-L1 expression:
23%
22%
29%
40%
0%
10%
20%
30%
40%
50%
60%
70%
All
PDL-L1+ (>25%)
PD-L1- (<25%)
PD-L1- (0%)
Objective Response Rate
(D10-20 q4/2w T1)
Durva + Treme
Goldmann J, ASCO 2017, abstract 9093; Antonia et al. Lancet Oncol. 2016;17(3):299-308
43
21
57
92
23
13
28
50
0
20
40
60
80
100
…
…
…
…
Nivo 3 + ipi 1 Q6/12W Nivo 3
Overall
<1%
≥1%
≥50%
PD-L1
expression









