
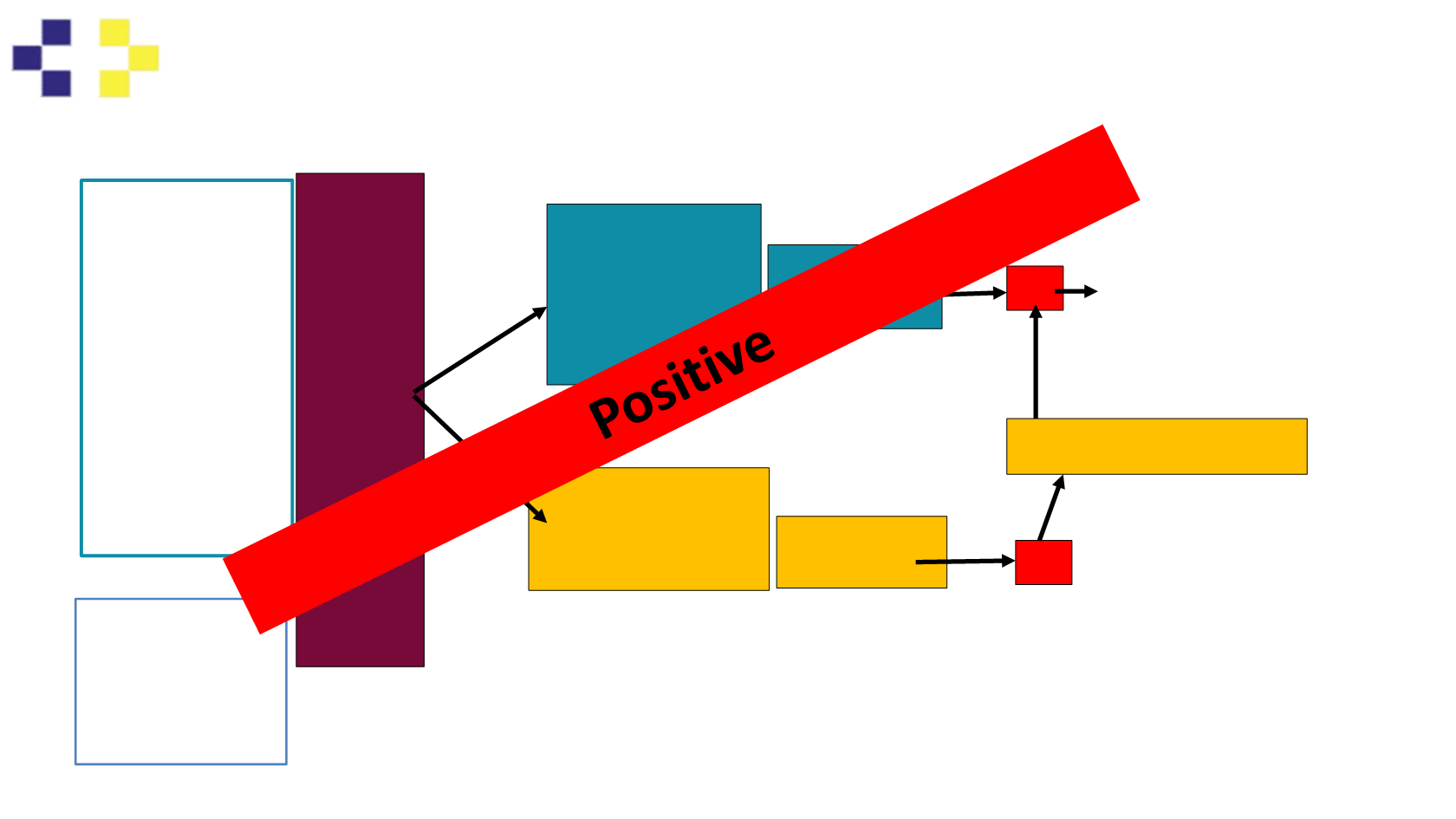
Study Design: Trial Diagram: KEYNOTE-189
Patients:
•
Metastatic non-
squamous NSCLC
•
First line metastatic
treatment
•
Measurable disease
•
ECOG PS 0-1
•
Tissue for
biomarker available
•
EGFR wild type
•
EML4/ALK or ROS-1
fusion negative
•
No known CNS
metastases
Stratify:
•
PDL1 prop score:
≥1%, <1%
•
Smoking status
•
cisplatin vs
carboplatin
R
A
N
D
O
M
I
Z
A
T
I
O
N
2:1
N=570
Carboplatin/Cisplatin
Pemetrexed
Pembrolizumab
200 mg Q3W
X4 cycles
Carboplatin/Cisplatin
Pemetrexed
+Saline
X4 cycles
Pemetrexed
Pembrolizumab
Pemetrexed
+Saline
PD
Pembrolizumab
PD
Primary Endpoint: PFS – target HR 0.7
Secondary Endpoints: OS, ORR, AE
Exploratory Endpoints: QoL
Off
Study









