
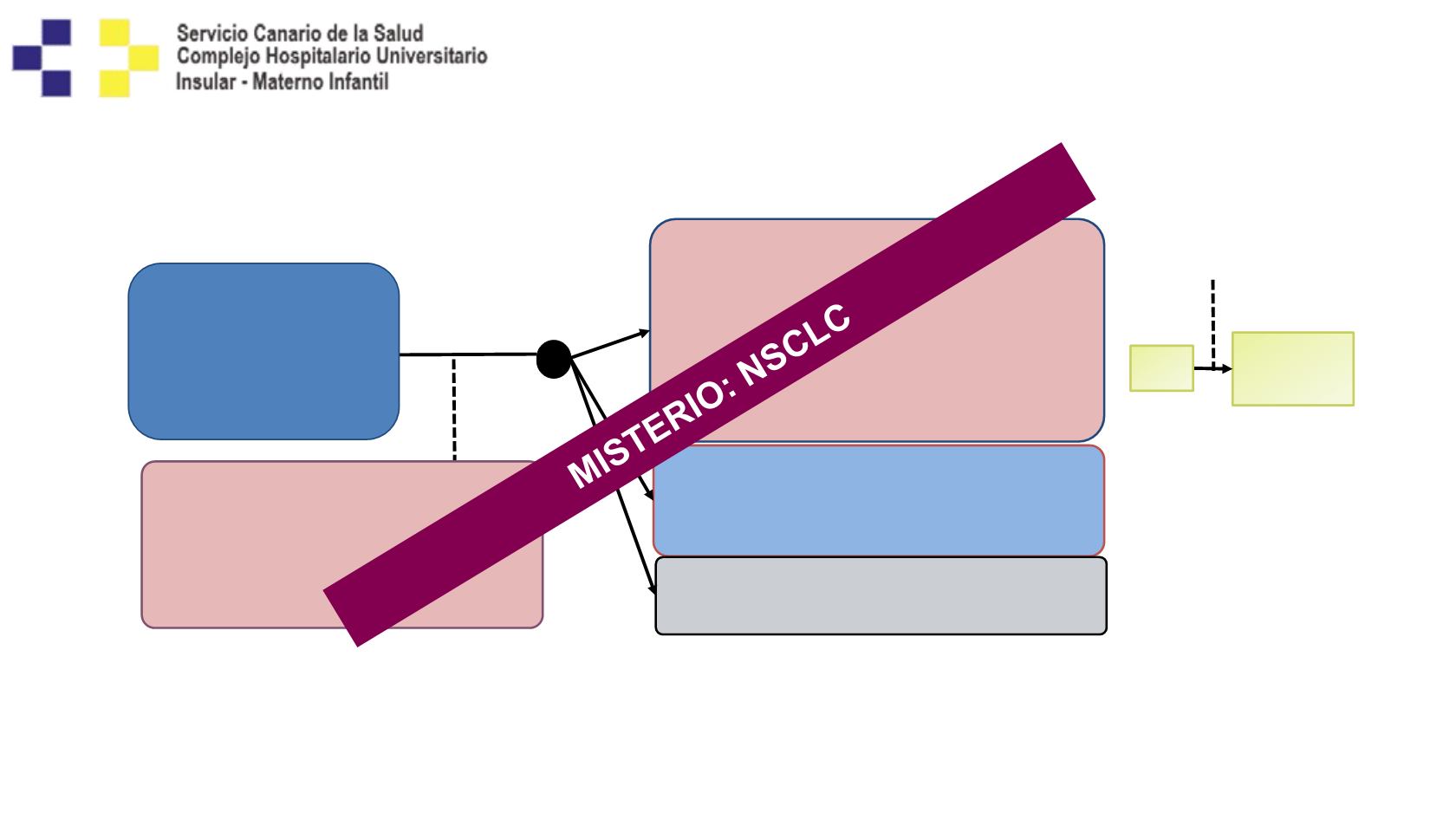
MYSTIC Study Design
•
Phase 3, randomized, open-label, multicenter, global study (>175 sites across Asia,
Australia, Europe, and North America)
1-3
FPCD: 3Q2015
LPCD: 2Q2016
Data anticipated:
mid-2017
Primary endpoint
1. PFS
in all patients and in
PD-L1(+) patients
2. OS in all patients
Secondary endpoints
•
ORR, DoR, PFS,
e
OS
f
•
Safety/tolerability, QoL
•
PK, immunogenicity
Follow-up
for OS
Subsequent
treatments
Arm 1
Durvalumab
iv 20 mg/kg q4w for 4 doses
+
Tremelimumab
iv 1 mg/kg q4w for 4 doses
Durvalumab
iv 20 mg/kg q4w starting on Week
16, for 9 doses (n=364)
Arm 2
Durvalumab
iv 20 mg/kg q4w for 13 doses,
for up to 12 months (n=364)
Arm 3
SoC
b
(n=364)
PD
R
1:1:1
n=1118
Patients with locally
advanced or metastatic
NSCLC (EGFR and
ALKwt,
a
Stage IV), 1L
(N=1850)
Stratification
1. PD-L1 status (positive vs negative)
2. Histology (squamous vs nonsquamous)









