
19
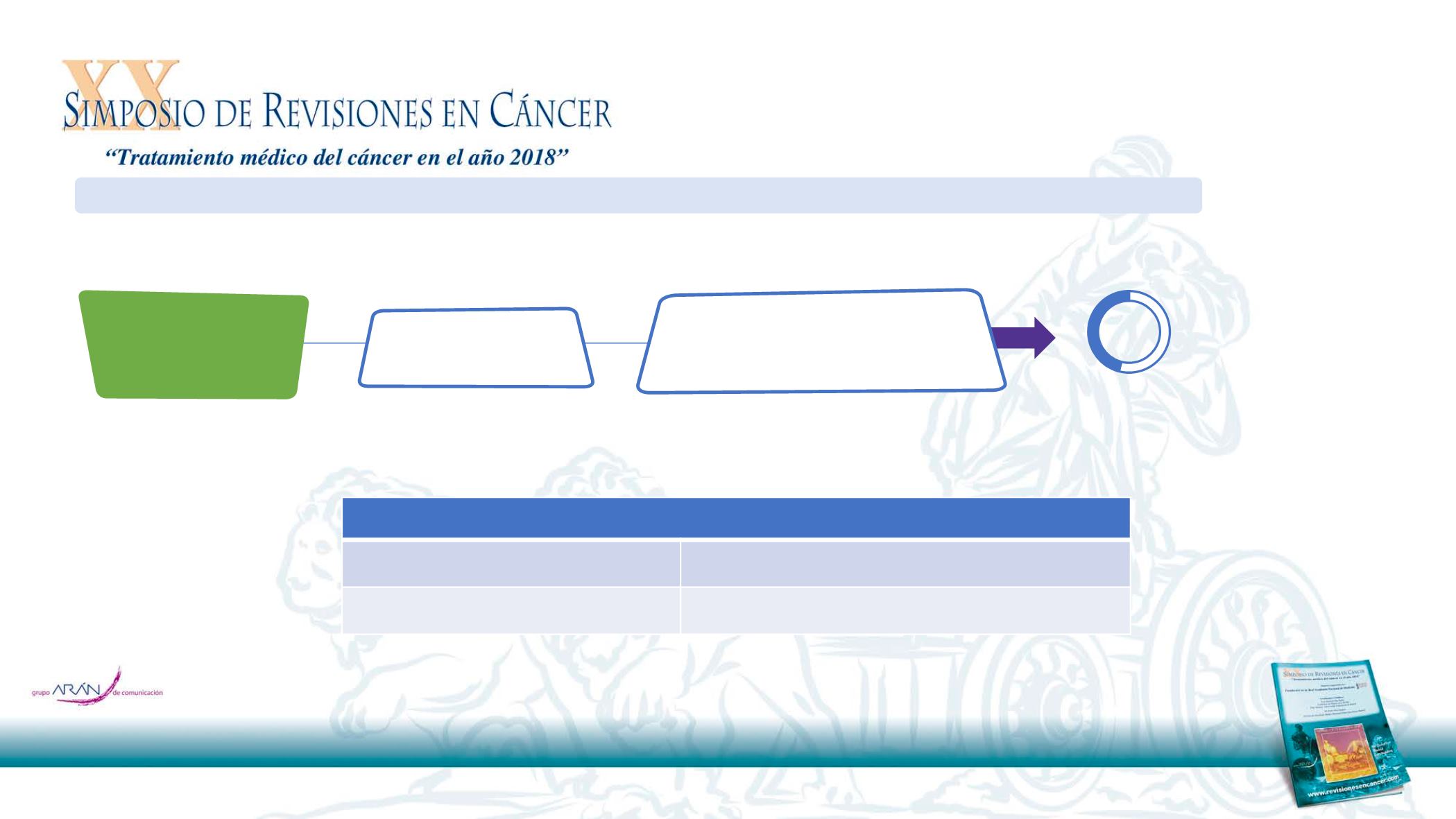
Estudio DIRECT (observacional): aceptabilidad en la práctica real
•
Guigay J, et al. Annals of Oncol 2016;27(Suppl. 6):Vi335 (Abstract 967P)
•
*Cetuximab was administered as a 400 mg/m
2
IV initial dose, then 250 mg/m
2
IV weekly + cisplatin OR carboplatin + 5-FU.
Patients received cisplatin (100 mg/m
2
IV, day 1) OR carboplatin (AUC 5 mg/mL/min IV, day 1) + 5-FU (1000 mg/m
2
IV, days 1–4). Error bars show 95% CI
Primary endpoint:
Frequency of patients with cetuximab RDI >80%
Cetuximab + platinum-
based CT*
Maintenance therapy (N=72)
Cetuximab monotherapy until disease
progression or unacceptable toxicity
Patients with 1
st
line R/M
SCCHN (N=157)
ECOG PS≥2 (18%)
Median number of cycles = 4
Median duration of maintenance =
15.8 weeks
46%
of patients received
cetuximab maintenance
Total prospective population (n=157)
12-month PFS, % (95% CI)
23.1 (14.0–33.5)
12-month OS, % (95% CI)
70.1 (57.5.–79.6)









