
14
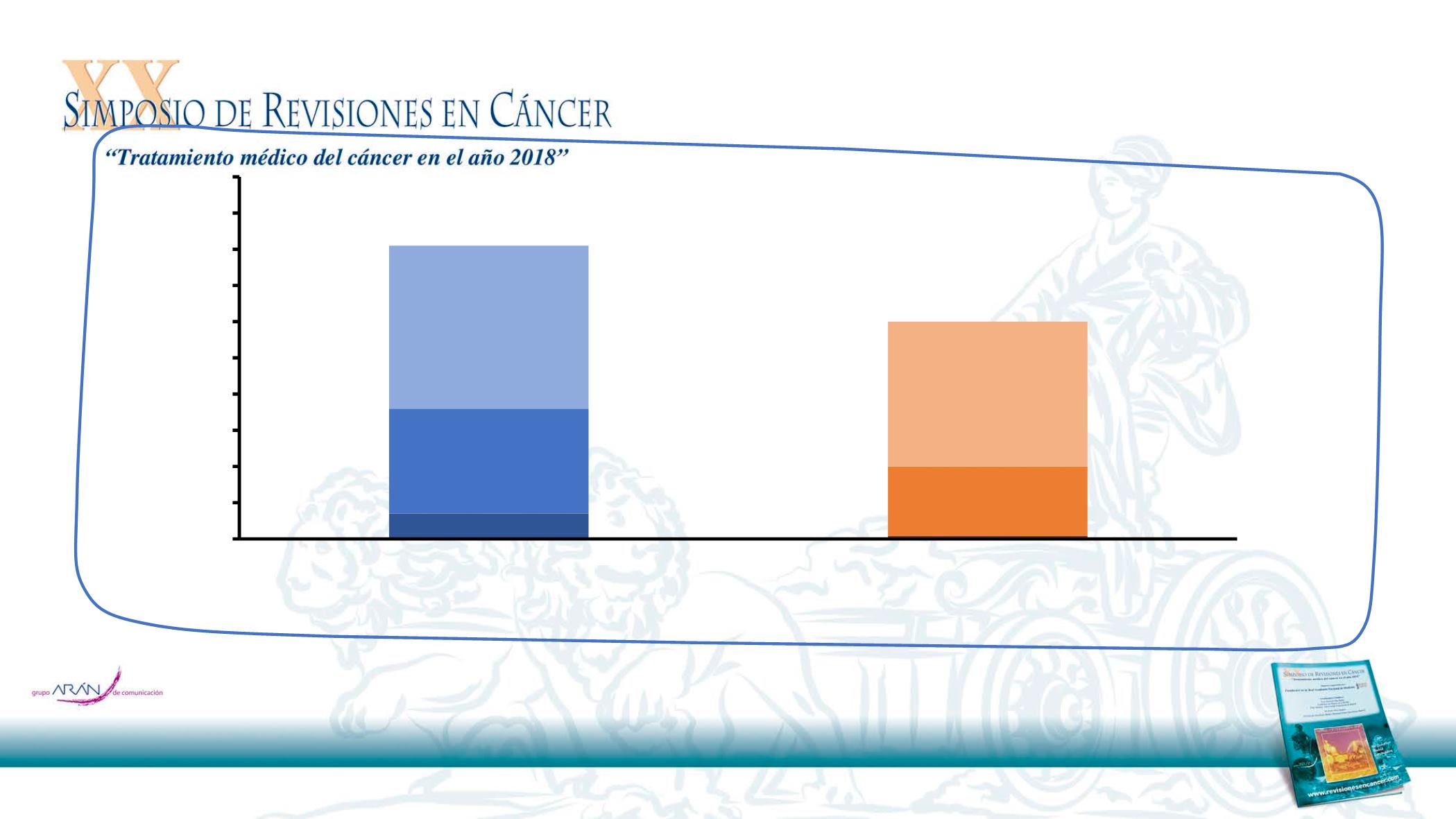
EXTREME: Tasa de Respuestas
•
1. Vermorken JB, et al. N Engl J Med 2008;359:1116–27
•
2. European Medicines Agency assessment report, Erbitux, 2008. Available from:
http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000558/WC500029118.pdf. Last accessed 7 October 2016
0
10
20
30
40
50
60
70
80
90
100
Cetuximab + CT
CT alone
CR, N=15
CR, N=2
ORR = 36%
ORR = 20%
DCR = 60%
DCR = 81%
(N=222)
(N=220)
Response (%)









