
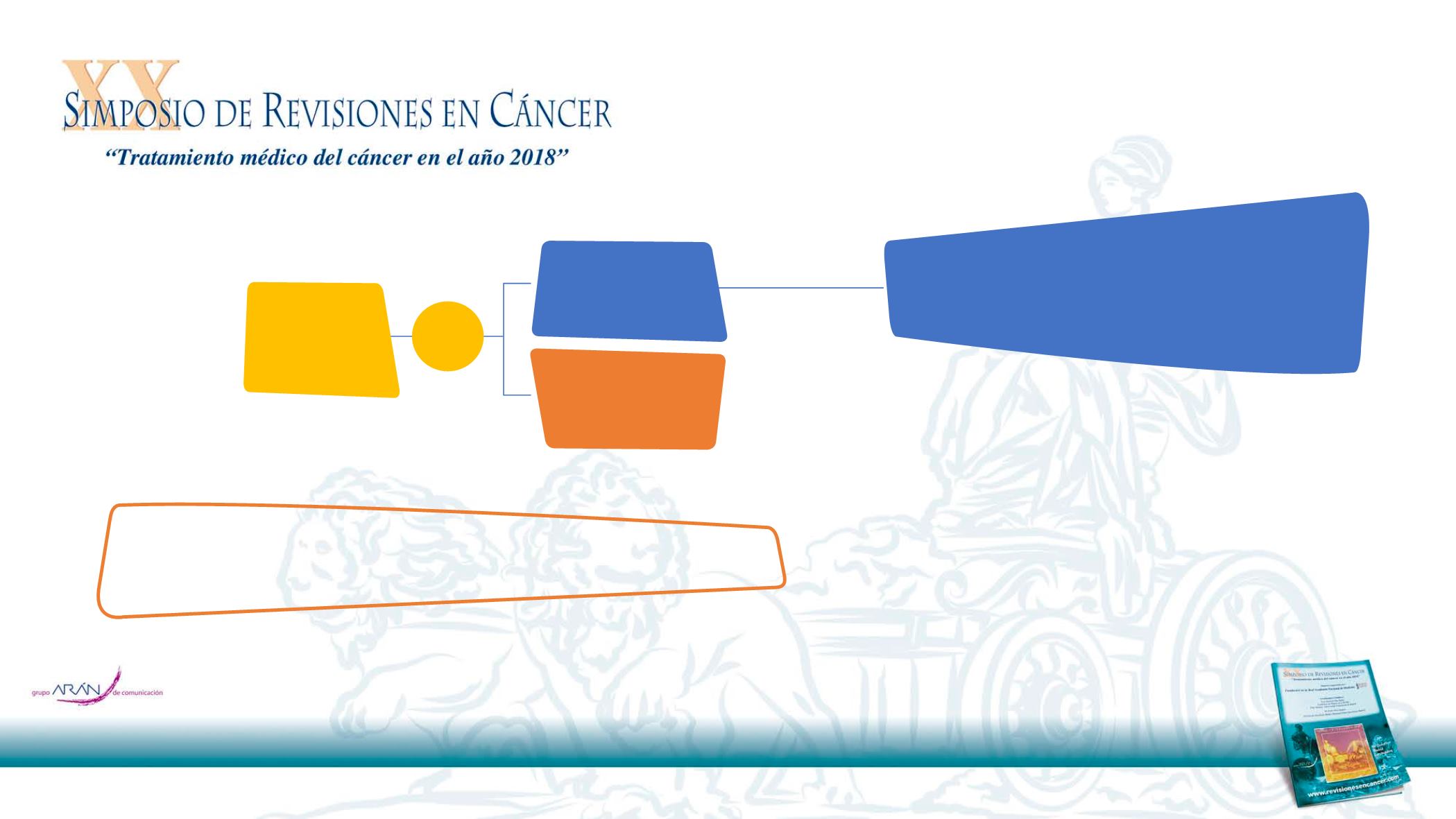
12
EXTREME
•
A pivotal, open-label, randomized, multi-center, phase III study in patients with 1st line R/M SCCHN
•
Vermorken JB, et al. N Engl J Med 2008;359:1116–27
•
*Cetuximab was administered as a 400 mg/m
2
IV initial dose, then 250 mg/m
2
IV weekly + cisplatin OR carboplatin + 5-FU; Patients received cisplatin (100 mg/m
2
IV, day 1) OR carboplatin
(AUC 5 mg/mL/min IV, day 1)† + 5-FU (1000 mg/m
2
IV, days 1–4) in 3-week cycles.
†
Cisplatin or carboplatin at investigator’s discretion
‡
Other secondary endpoints not listed
•
AUC, area under the curve; IV, intravenous; KPS, Karnofsky performance score; CR, complete response; SD, stable disease
Primary endpoint:
Overall survival
Key secondary endpoints:
PFS, ORR and safety
‡
R
1:1
Up to 6 cycles
(18 weeks)
If PR, CR, SD
Stratification
•
KPS (<80%
vs ≥80%)
•
Previous CT
(receipt vs non-
receipt)
Cetuximab +
platinum-
based CT* (N=222)
CT alone
Platinum-based CT
†
(N=220)
Maintenance
Cetuximab monotherapy
until disease progression or unacceptable
toxicity
Patients with 1st
line R/M SCCHN
(N=442)









