
9
*Treatment decision based on performance status
†
Platinum-based CT, consisting of
cisplatin/carboplatin + 5-FU; ;
ǂ
Pembrolizumab and nivolumab are not approved in the EU
†
CT, chemotherapy; LA, locally advanced; PD, progressive disease;
RT, radiotherapy
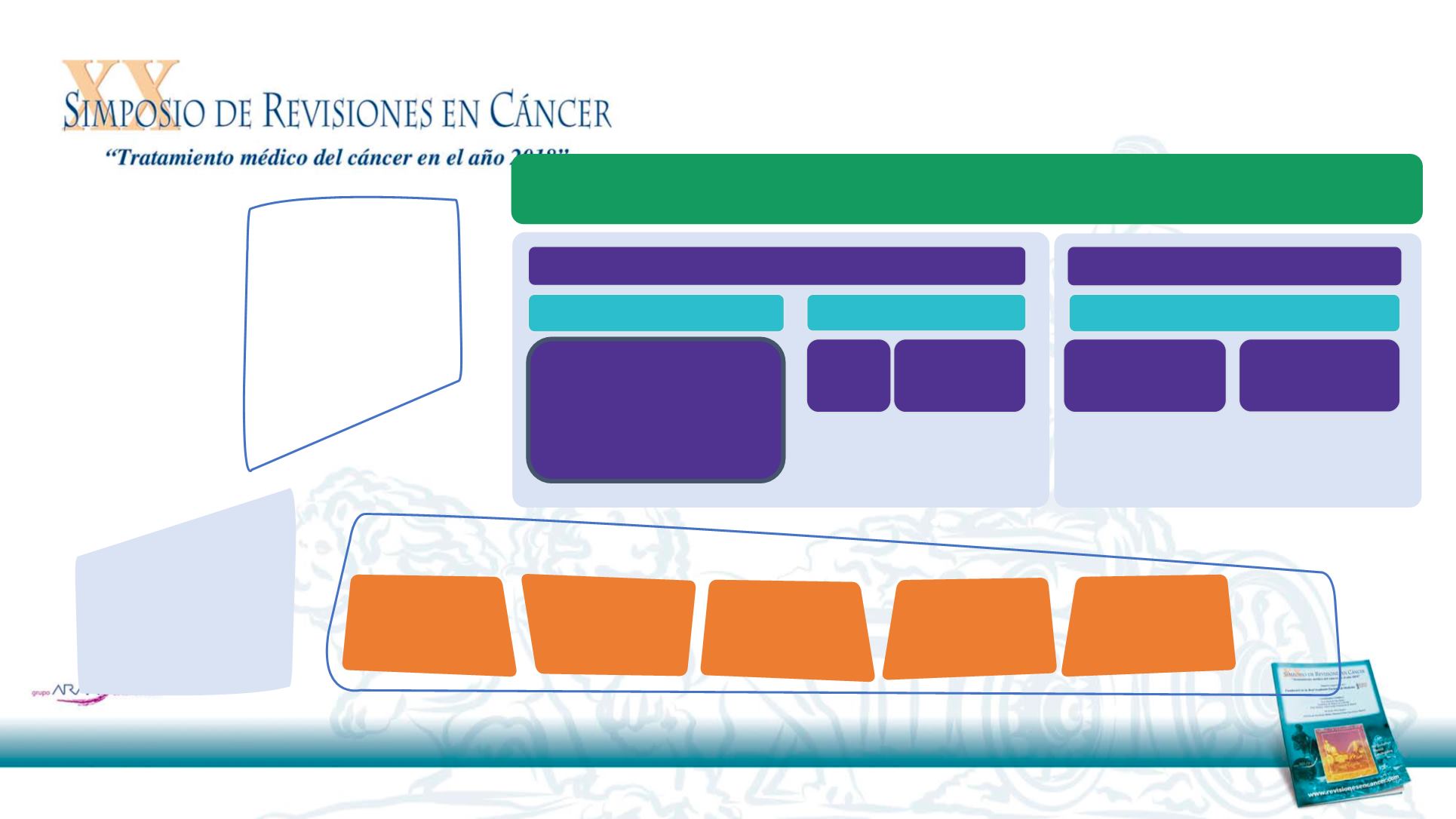
R/M SCCHN
Objective: Curative
Patients fit
Best
supportive
care
EXTREME
1,2
:
Platinum based-CT
†
+
cetuximab followed by
cetuximab
maintenance until PD
1L
*
2L+
Taxanes
Clinical trial
Methotrexate
Patients unfit
Cetuximab
Objective: Palliative
Objective: Palliative
Cetuximab +
taxanes
2
RT
Surgery
1. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers V1 2017
2. Docampo et al Clinical and Translational Oncology 2017
3. Ferris RL, et al. N Engl J Med 2016;375 (19):1856–67
4. Bauml J, et al.2016 (Abstract No. 6011)
Taxanes
Clinical trial
Methotrex te
Cetuximab
Checkpoint
inhibitor
ǂ
3,4









