
20
Combinaciones con taxanos: The GORTEC 2008-03 study
•
1. Guigay J, et al. Ann Oncol 2015;26:1941–7
•
2. Erbitux SmPC, June 2014
•
*
Taxanes are not approved for R/M SCCHN in the EU;
†
Cetuximab administered every 2 weeks at 500mg/m
2
in the maintenance phase
(Note: off-label use
2
)
ITT, intent-to-treat
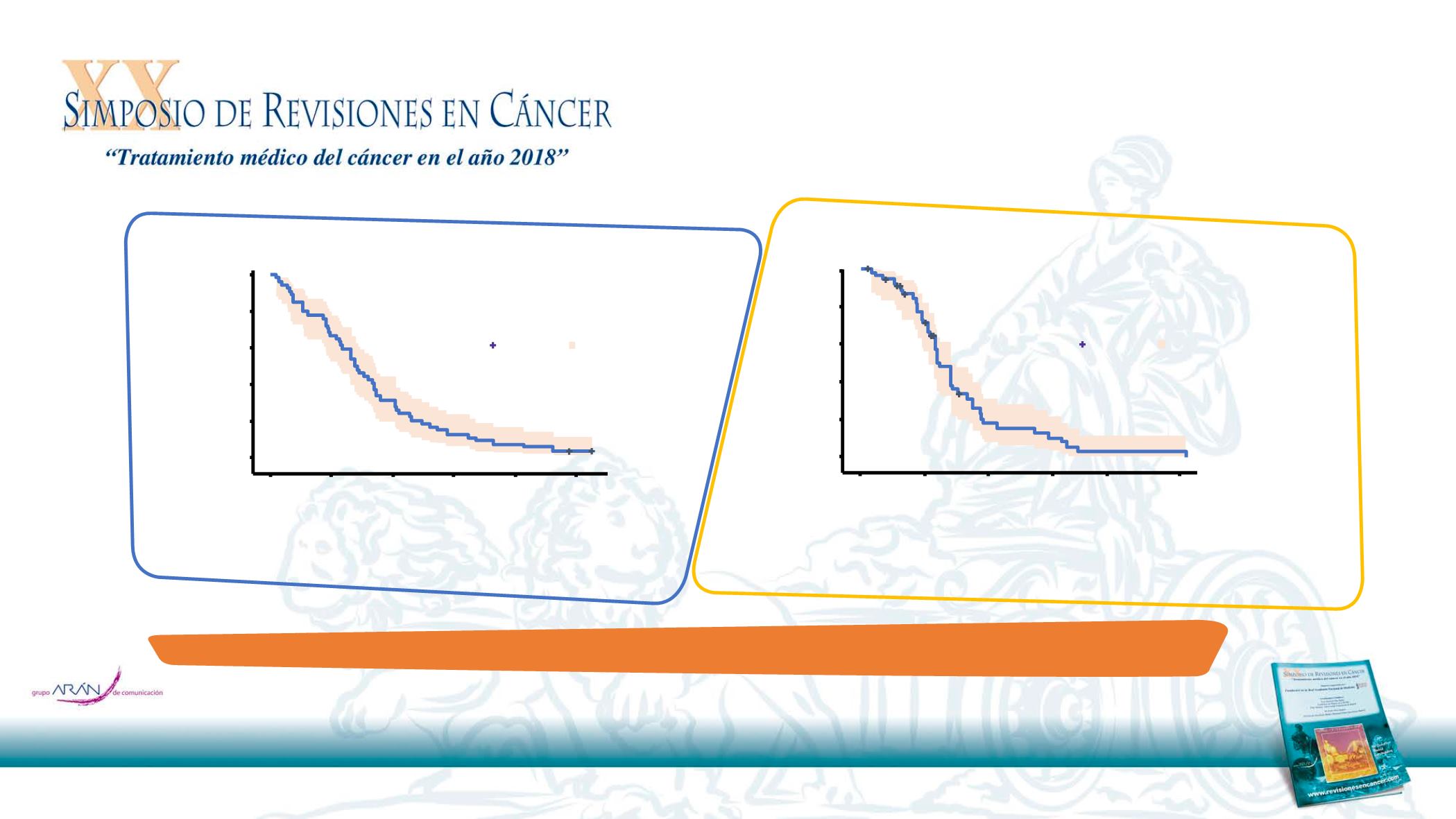
Overall survival (ITT population)
Progression-free survival (ITT population)
Primary endpoint:
Objective response rate at Week 12: 44% (95% CI: 30.9–58.6)
1.0
0.8
0.6
0.4
0.2
0.0
0
10
20
30
40
50
43 32 17 11 7 5 3 2 0
Time (months)
Probability of overall survival
Median OS = 14.0 months
(95%CI: 11.3
–17.0)
Time (months)
0
5
12
18
24
30
36
42
48
54
No. at risk
54
43
32
17
11
7
5
3
2
2
Censored 95% CI
1.0
0.8
0.6
0.4
0.2
0.0
0
5
10
15
20
25
21
6
1
0
Time (months)
Probability of Progression-
free survival
Median PFS= 6.2 months
(95% CI: 5.4
–7.2)
Time (months)
0
6
12
18
26
No. at risk
54
21
6
1
1
Censored 95% CI
•
A prospective, open-label, multi-center, phase II study in patients with 1st line R/M SCCHN treated with docetaxel
*
in combination with cisplatin
and cetuximab (up to 4 cycles) followed by cetuximab maintenance until PD (TPEx; N=54)
†









