
22
IDR durante el mantenimiento con CTX:
estudios EXTREME
1
and DIRECT
2
•
1. Vermorken JB, et al. N Engl J Med 2008;359:1116–27
•
2. Guigay J, et al. ESMO 2016 (Abstract 967P)
EXTREME
•
Cutaneous toxicities were observed in 20/219 (9.1%) of EXTREME study patients (safety population; grade ≥3)
1
•
and 25/72 (34.7%) of DIRECT study patients (maintenance phase; all grades)
2
•
Neutropenia was the most common non-cutaneous toxicity observed in both the EXTREME and DIRECT study patients
1,2
0
20
40
60
80
100
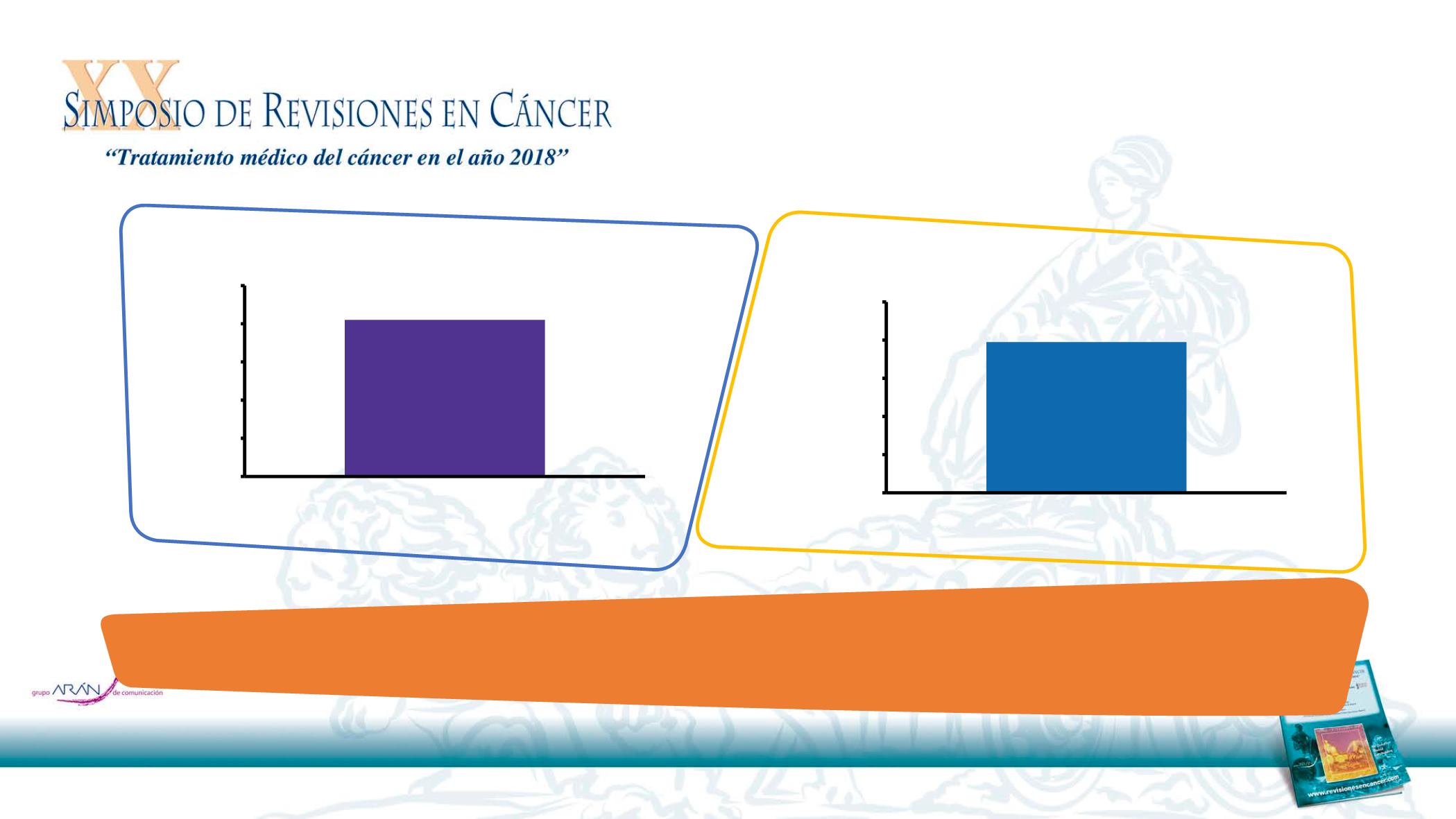
Cetuximab maintenance
therapy (N=100)
Patients (%) with cetuximab RDI
≥80%
82%
DIRECT
0
20
40
60
80
100
Cetuximab maintenance
therapy (N=72)
Patients (%) with cetuximab RDI
≥80%
79%









