
21
CTX + paclitaxel + platino-based CT: estudio CSPOR-HN02
An open-label, multi-center, phase II study in patients with 1st line R/M SCCHN treated with paclitaxel
*
in combination with carboplatin and
cetuximab (up to 6cycles) followed by cetuximab maintenance until PD (PCE regimen; n=45)
•
Tahara M, et al. ASCO 2016 (Abstract No. 6026)
•
*
Taxanes are not approved for R/M SCCHN in the EU;
†
Cetuximab was administered as a 400 mg/m
2
initial dose, then 250 mg/m
2
weekly. Patients
received paclitaxel (100mg/m
2
, Days 1 and 8) carboplatin (AUC 2.5 mg/mL/min. Days 1 and 8) in 3-week cycles
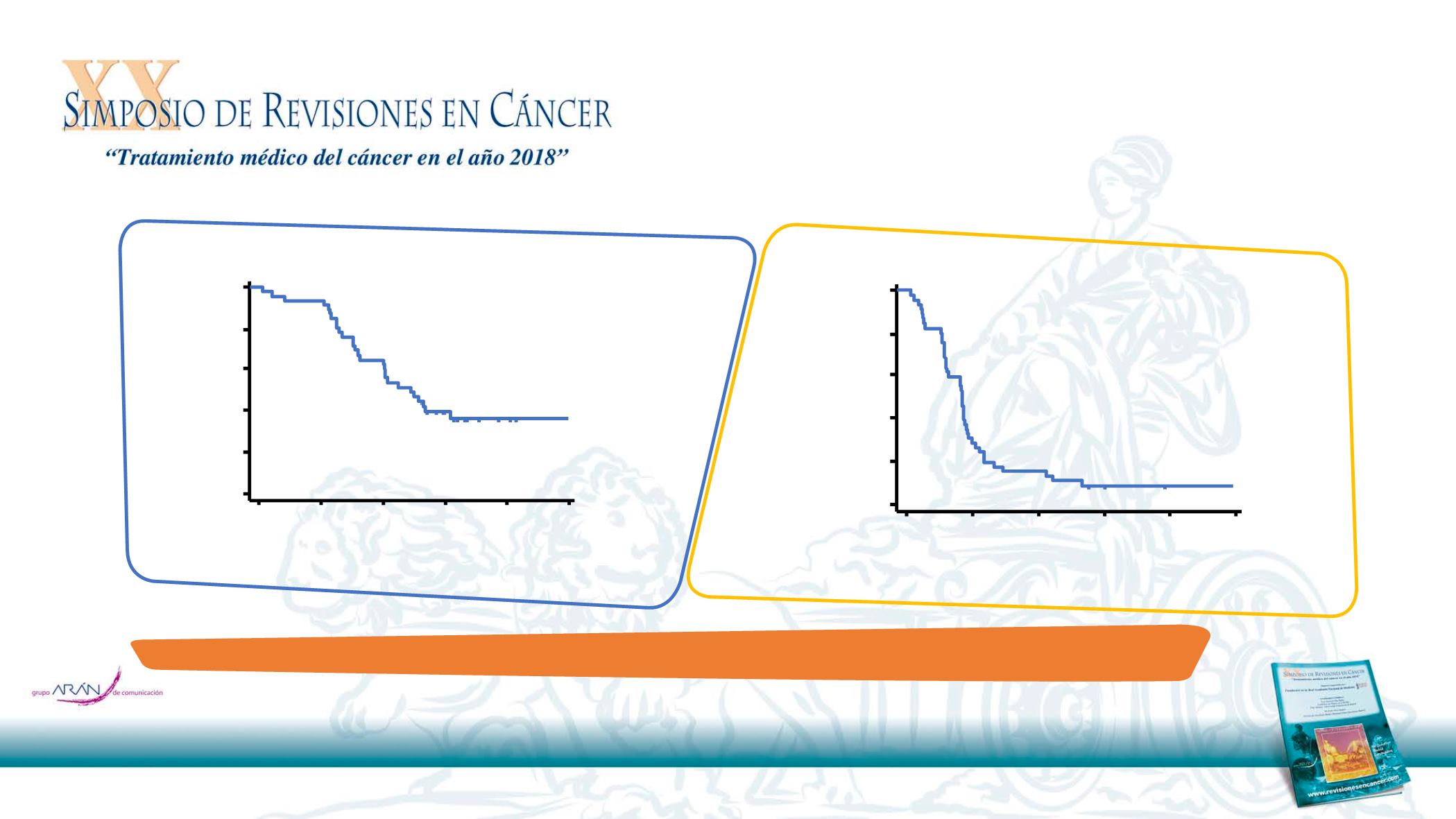
Overall survival
Progression-free survival
Median PFS: 5.2 months
(95% CI:
3.9‒5.6)
Primary endpoint:
Overall response rate 40% (95% CI: 25.7–55.7)
Median OS: 14.7 months
(95% CI: 9.8‒not reached)
100
80
60
40
20
0
0
6
12
18
24
30
Overall survival (%)
Time from registration (months)
45
42
29
13
3
1
Number at risk
100
80
60
40
20
0
0
6
12
18
24
30
Progression-free
survival (%)
Time from registration (months)
45
14
7
3
1
1
Number at risk









