
•
Phase 1/2, multiple cohort, max 2 lines
therapy
o
Virus-associated tumours
o
ECOG 0-1
o
Until progression or unacceptable toxicity
•
Primary Endpoint:
-Objective Response
-Safety
o
Tumor evaluation q8w Y1, q12w Y2 and beyond
Anti-PD-1: Nivolumab 240mg q2w
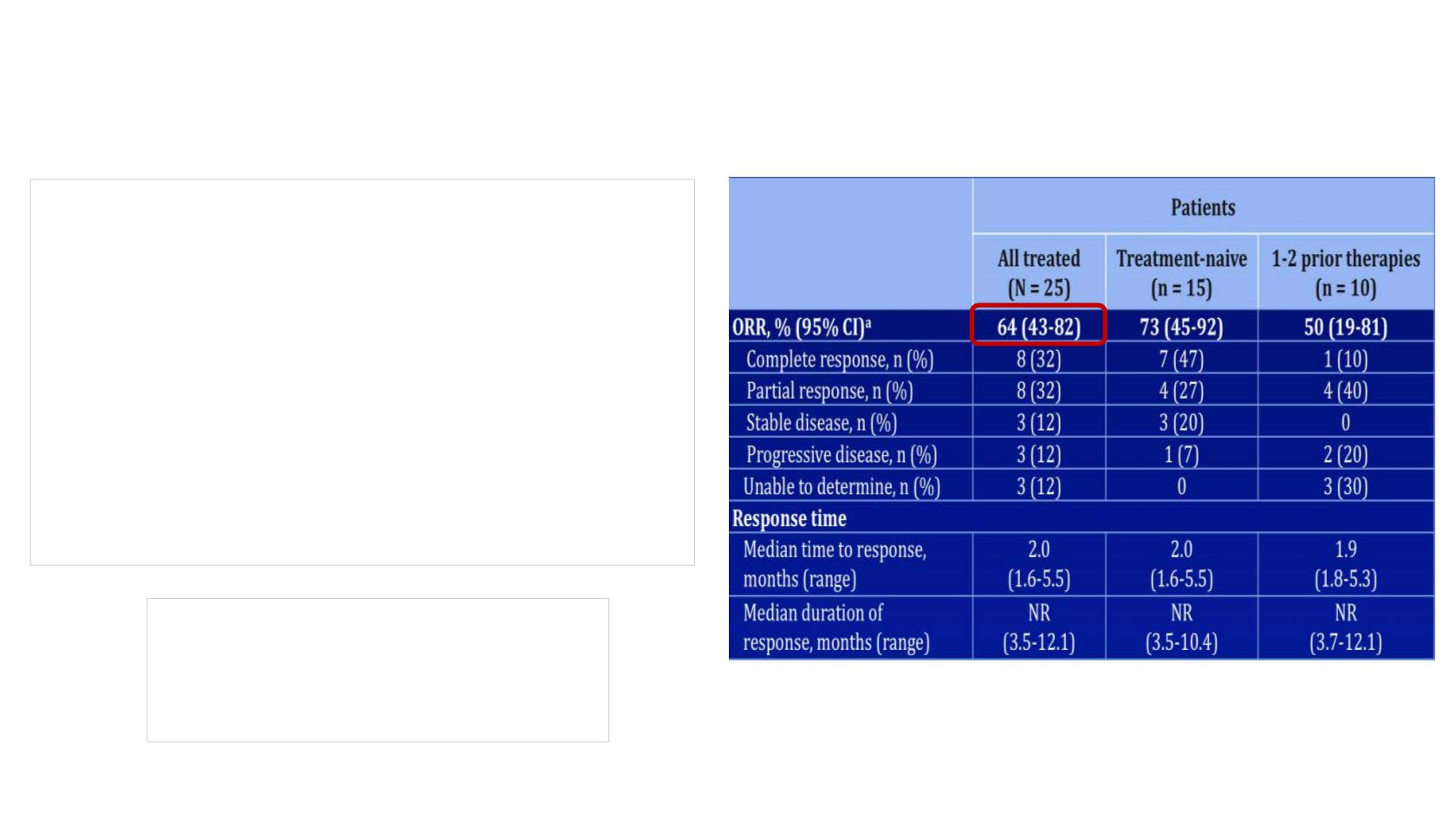
N= 25 (Merkel Ca)
60% no previous treatment
40% 1 or 2 prior therapies
Topalian et al. AACR 2017 abstr CT074









