
Anti-PD-1: Pembrolizumab 2mg/kg q3w
•
Phase 2, multi-center, First Line
o
Unresectable or M1 Merkel Carcinoma
o
ECOG 0-1
o
Up to 2 years or unacceptable toxicity
•
Primary Endpoint:
Objective Response
o
Tumor evaluation q12w 1st, q9w x 1Y, q12w in Y2
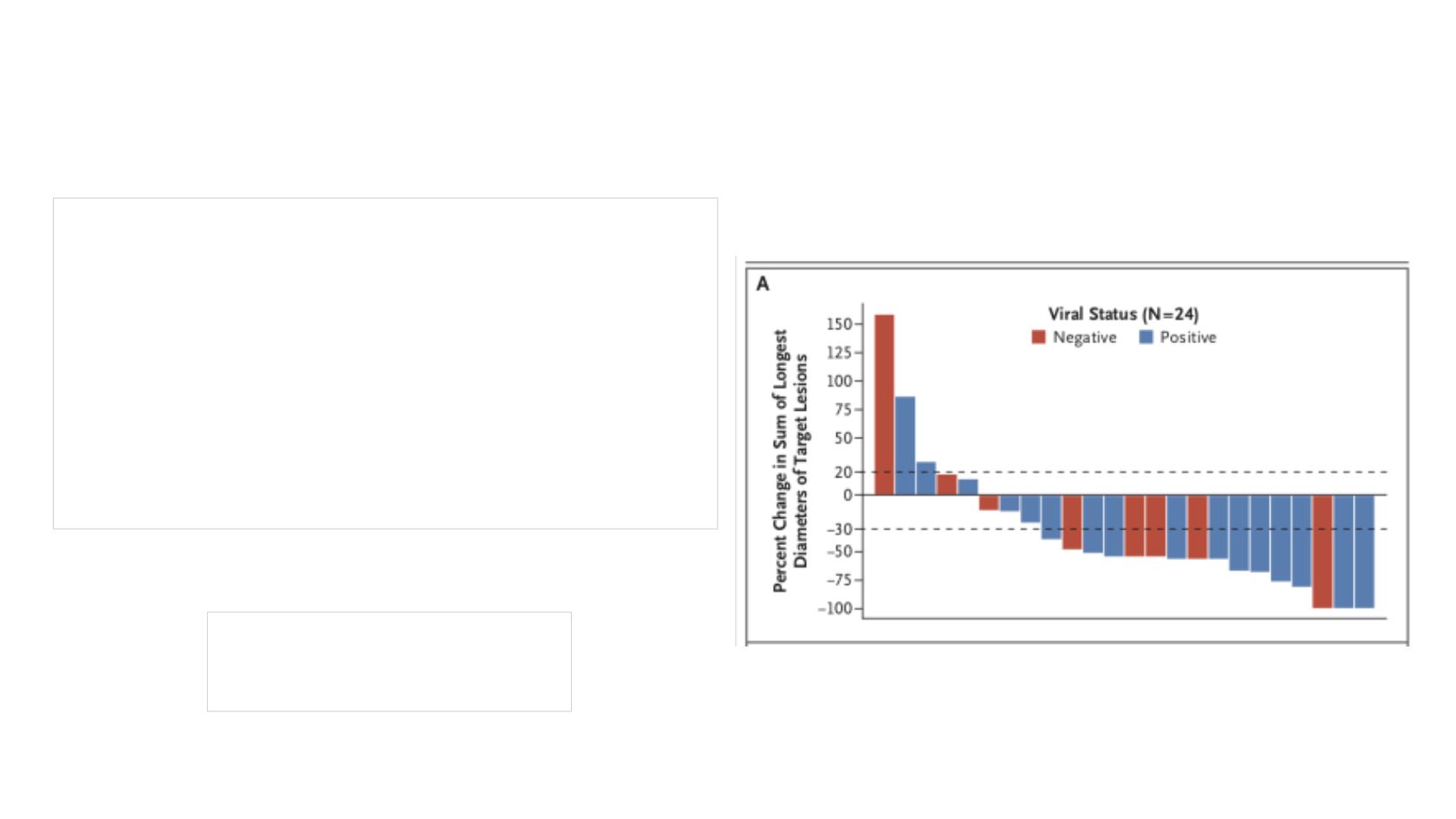
N= 26
III= 8% IV= 92%
RR 56% (4CR, 10 PR)
RR 10/16
RR 4/9
Nghiem P et al. NEJM 2016
MCPyV no impact on response to anti-PD-1









