
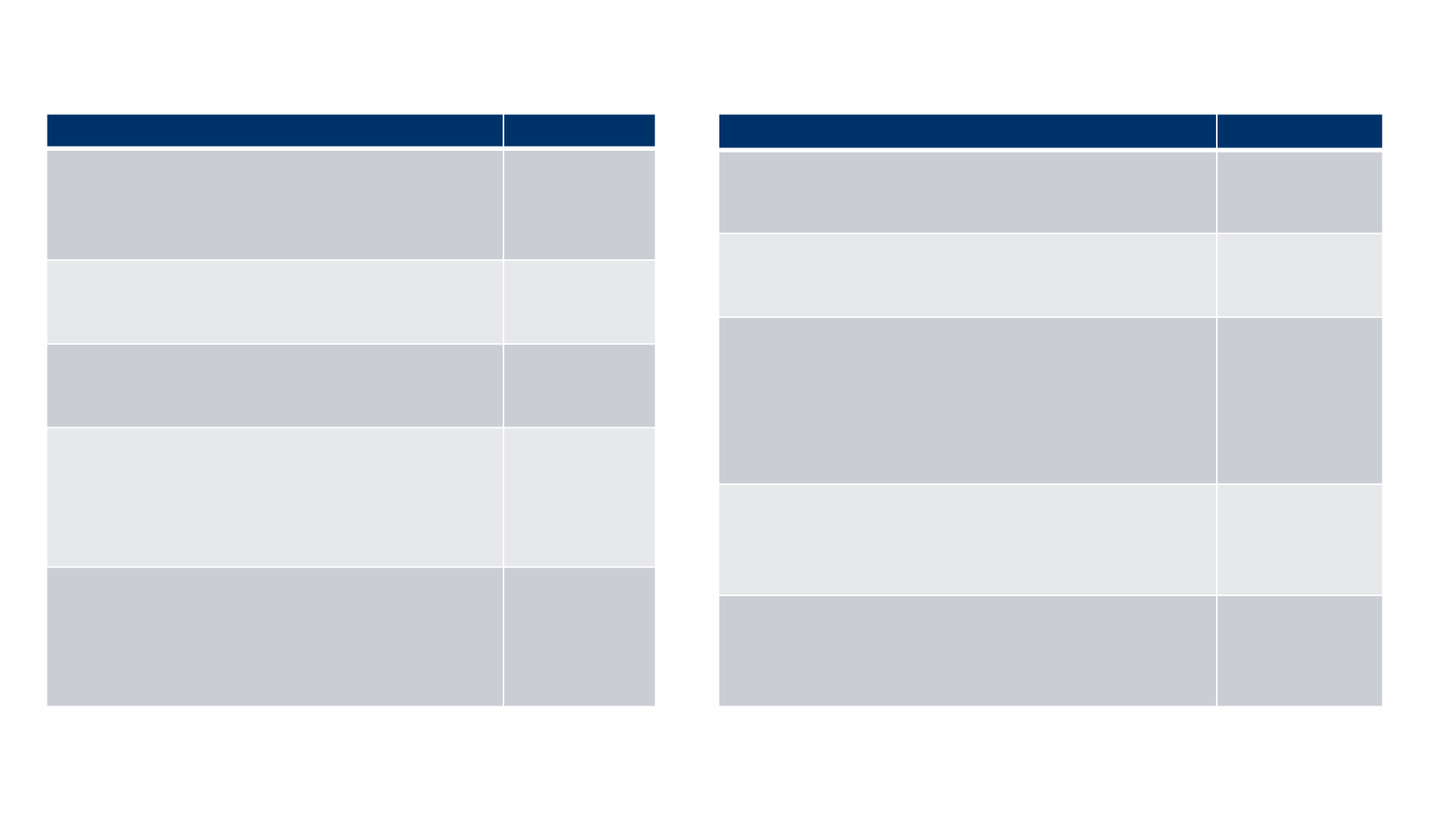
Patient characteristics: Part A, 2L
* Metastases not isolated to lymph nodes, skin, or soft tissue.
†
PD-L1 positivity was defined by a threshold level of ≥1% positive tumor cells of any intensity detected by immunohistochemistry (IHC) using a
proprietary assay (Dako PD-L1 IHC 73-10 pharmDx; Dako, Carpinteria, CA, USA).
ǂ
MCPyV large T-antigen expression was assessed by IHC using a specific monoclonal antibody clone CM2B4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Kaufman HL, et al. Lancet Oncol. 2016;17(10):1374-85; Kaufman.AACR 2017
Characteristic (N=88)
n (%)
Age
<65 years
≥65 years
Median
, years (range)
22 (25.0)
66 (75.0)
72.5 (33-88)
Sex
Male
Female
65 (73.9)
23 (26.1)
ECOG PS
0
1
49 (55.7)
39 (44.3)
Site of primary tumor
Skin
Lymph node
Other
Missing
67 (76.1)
12 (13.6)
2 (2.3)
7 (8.0)
Number of prior systemic anticancer
treatments
1
2
≥3
52 (59.1)
26 (29.5)
10 (11.4)
Characteristic (N=88)
n (%)
Visceral disease at study entry*
Yes
No
47 (53.4)
41 (46.6)
Lymph node disease only
Yes
No
19 (21.6)
69 (78.4)
Sum of target lesion diameters (SLD) at
baseline
≤Median SLD
>Median SLD
Not evaluable
Median SLD, mm (range)
39 (44.3)
38 (43.2)
11 (12.5)
79.0 (16-404)
Tumor PD-L1 expression
†
Positive
Negative
Not evaluable
58 (65.9)
16 (18.2)
14 (15.9)
Tumor MCPyV status
ǂ
Positive
Negative
Not evaluable
46 (52.3
)
31 (35.2)
11 (12.5)









