
Patients with aggressive course of disease –
Results from LUME Lung 1 and Checkmate 057
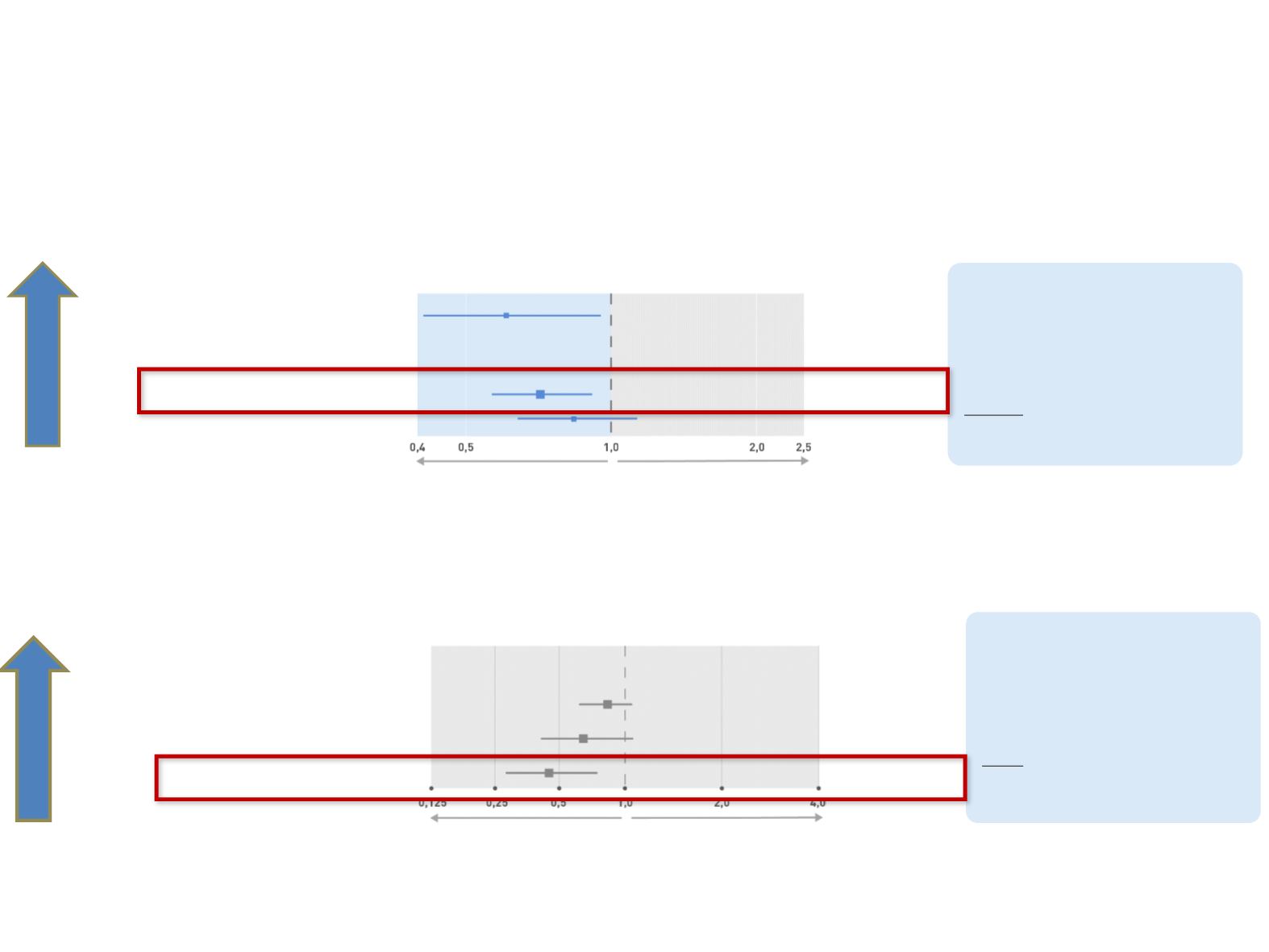
LUME-Lung 1: OS benefit for patients with adenocarcinoma
1
1. Reck M et al. Lancet Oncol. 2014;15(2):143–155. │ 2. Borghaei H et al. N Engl J Med. 2015;373(17):1627–39.
n
% of patients
Hazard Ratio
Time since completion of prior
therapy
< 3 months
364
63 %
0,85 (0,67–1,08)
3-6 months
115
20 %
0,69 (0,44–1,08)
> 6 months
103
18 %
0,46 (0,27–0,79)
n
% of patients
Hazard Ratio
1st line refractory
117
18 %
0,62 (0,41–0,94)
Time since start of 1
st
line until PD
<9 months
405
62 %
0,75 (0,60–0,92)
≥ 9 months
246
37 %
0,89 (0,66–1,19)
LUME-Lung 1 (Nintedanib + Docetaxel vs. Docetaxel + Placebo)
Favors
Nintedanib + Docetaxel
Favors Docetaxel
HR
Nivolumab:
Increasing benefit with
later progression
CheckMate 057 (Nivolumab vs. Docetaxel)
CheckMate 57: OS benefit for patients with non.squamous NSCLC
2
Favors Nivolumab
Favors Docetaxel
HR
Nintedanib + Docetaxel:
Increasing benefit with
earlier progression
Earlier Progress
Earlier Progress









