
Treatment until
disease
progression
or unacceptable
toxicity
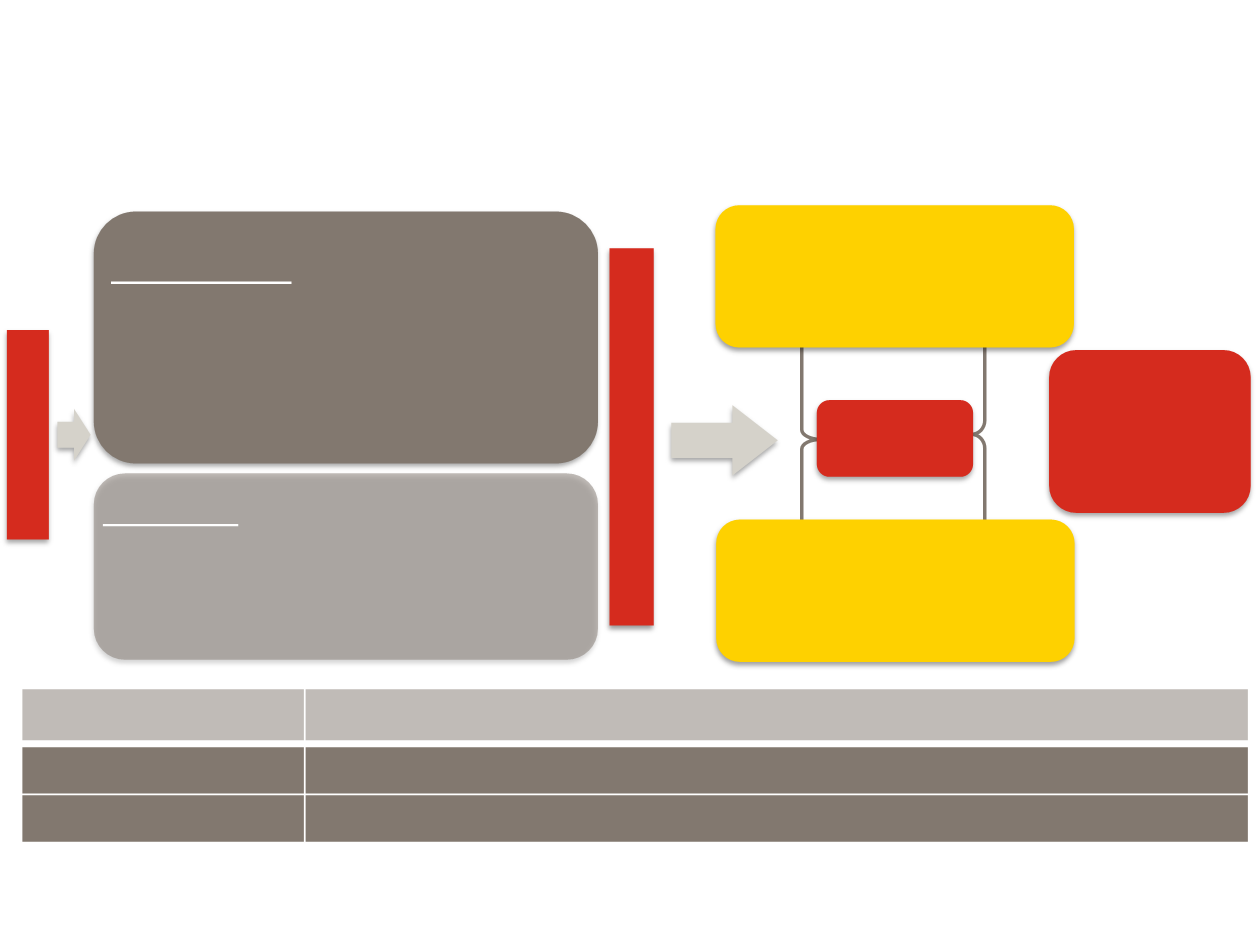
Inclusion Criteria
•
Stage IV NSCLC progressing on or after 1
platinum-based regimen
•
Prior Bev allowed
•
Squamous or nonsquamous histologies
•
ECOG PS 0/1
Placebo
+
aDocetaxel 75 mg/m2
N=625
Every 3
weeks
aDocetaxel 60 mg/m2 in Korea and
Taiwan
R
A
N
D
O
M
I
Z
E
1:1
Study design
Phase 3, randomized, multisite study of ramucirumab or placebo plus docetaxel following progression on or
after a platinum-based regimen
Primary endpoint
Overall survival
Secondary endpoints
Progression-free survival, overall response rate, safety, patient-reported outcomes
Stratification
•
Geography (East Asia or other)
•
ECOG PS
•
Gender
•
Prior maintenance therapy (Y or N)
S
C
R
E
E
N
N=1253
Ramucirumab 10 mg/kg
+
aDocetaxel 75 mg/m2
N=628
REVEL study - phase 3 study in NSCLC
(2nd-line: docetaxel plus Ramucirumab/placebo)
Garon E, et al. Lancet 2015









