
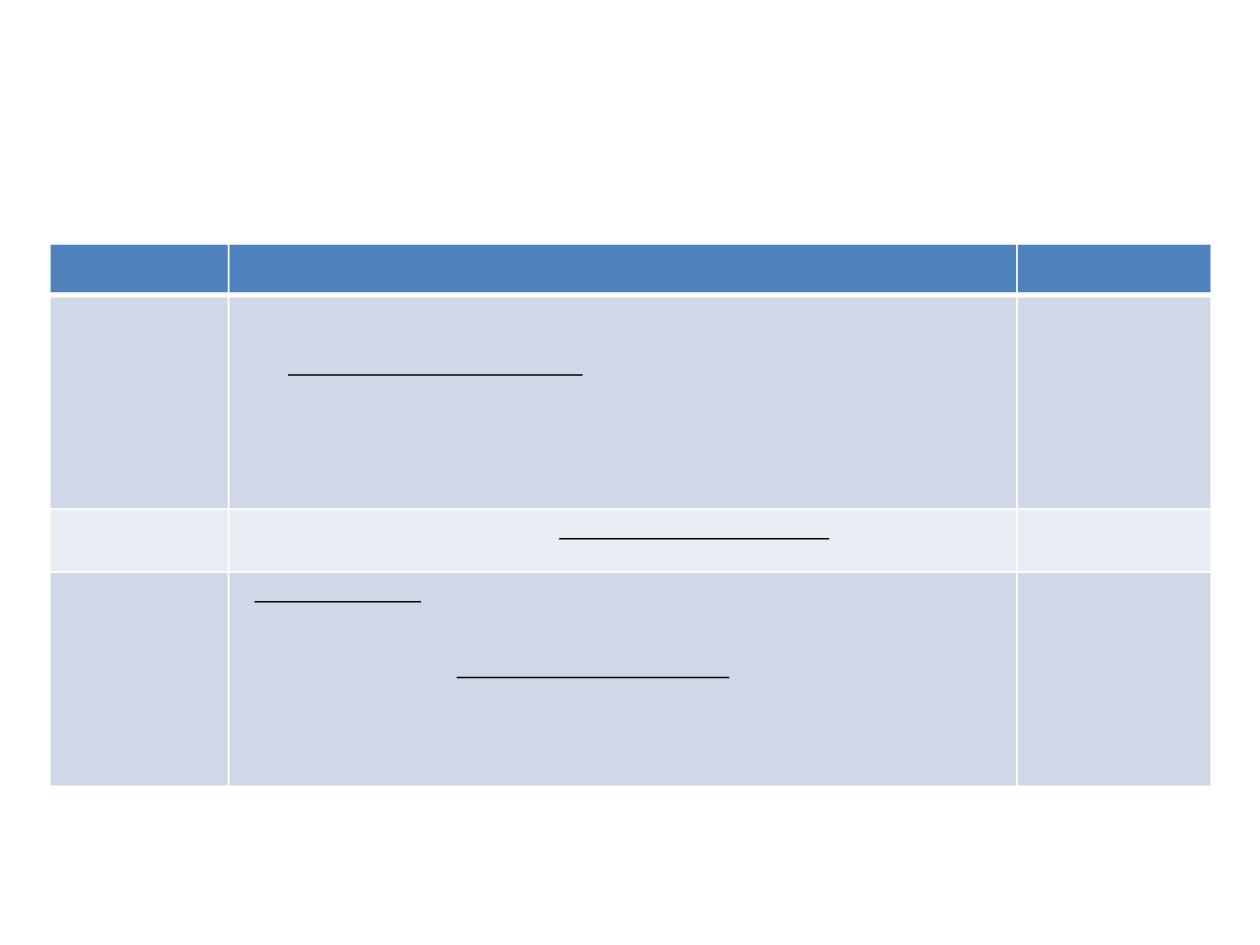
Trial
Exclusion criteria
OS (months)
CheckMate 017
and 057
Any other serious or uncontrolled medical disorder,
active infection
, physical
exam finding, laboratory finding, altered mental status, or psychiatric condition
that, in the opinion of the investigator, would limit a subject’s ability to comply with
the study requirements, substantially increase risk to the subject, or impact the
interpretability of study results.
CM 017
Nivo = 9.2
Docetaxel = 6
CM 057
Nivo = 12.2
Doc = 9.5
KEYNOTE-010
Has an
active infection
requiring intravenous systemic therapy.
Pembro = 10.5
Doc = 8.6
OAK
• Severe infections within
4 weeks prior to randomisation
including but not
limited to hospitalisation for complications of infection, bacteraemia or severe
pneumonia.
• Received therapeutic oral or intravenous antibiotics
within 2 weeks prior to
randomisation.
- Patients receiving prophylactic antibiotics (eg, for prevention of a urinary tract
infection or chronic obstructive pulmonary disease) are eligible
Atezo = 13.8
(15.6 Non-Sq)
Doc = 9.6 (11.2
non-Sq)
Exclusion criteria for anti-PDL1 and anti-PD1 therapies in
previously treated NSCLC regarding the use of antibiotics









