
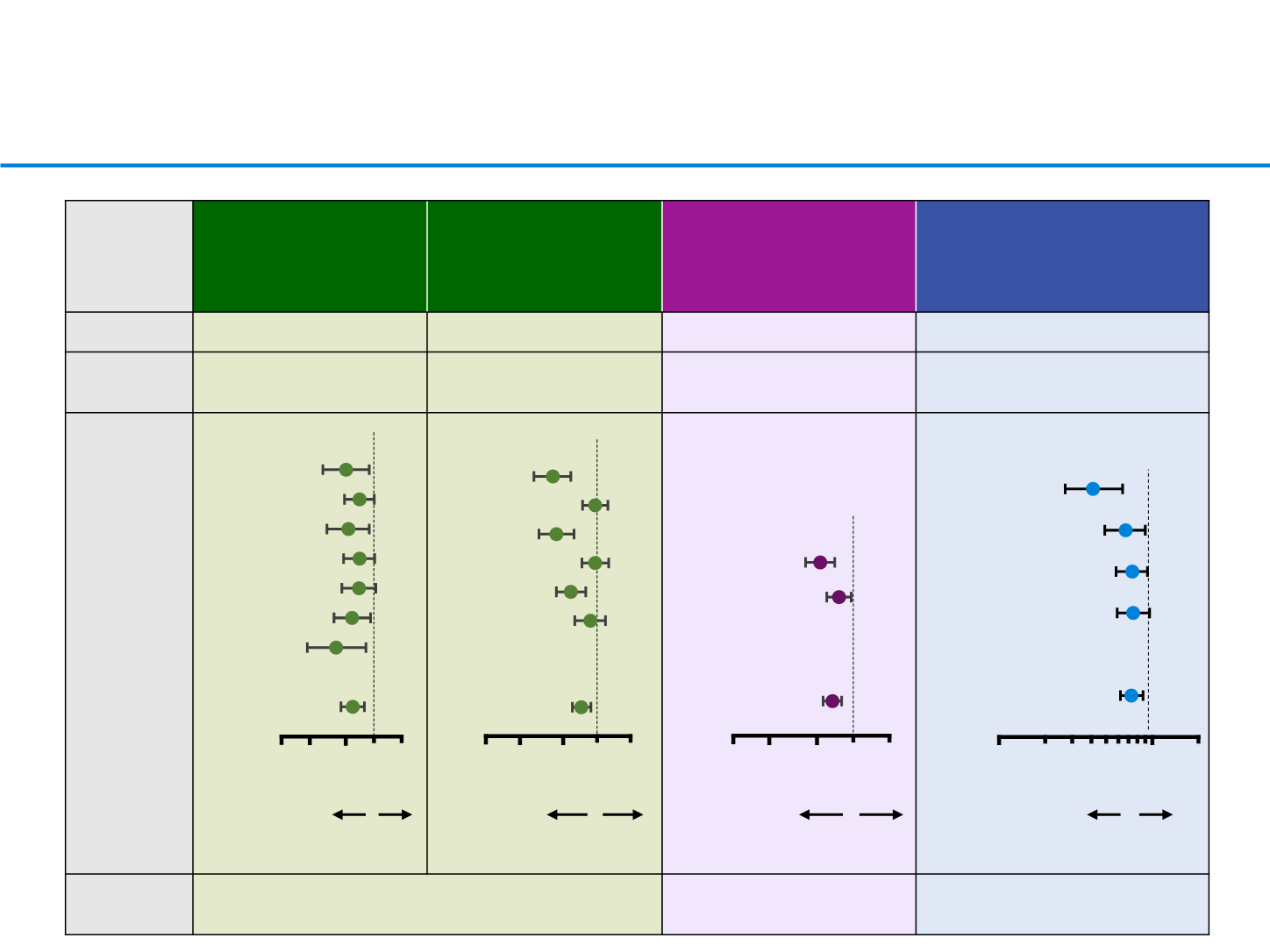
Immunotherapy in previously treated patients:
efficacy by PD-L1 status
*TC3 or IC3: ≥50% of TCs or ≥10% of ICs; TC2/3 or IC2/3: ≥5% of TCs or ICs;
TC1/2/3 or IC1/2/3: ≥1% of TCs or ICs; TC0 and IC0: <1% of TCs and ICs
1. Brahmer, et al. N Engl J Med 2015; 2. Borghaei, et al. N Engl J Med 2015
3. Herbst, et al. Lancet 2015; 4. Barlesi, et al. ESMO 2016
CheckMate 017 (phase
III)
1
2L nivo vs doc (n=272)
CheckMate 057 (phase
III)
2
2/3L nivo vs doc
(n=582)
KEYNOTE-010 (phase
II/III)
3
≥2L pembro
¶
vs doc
(n=1,033)
OAK (phase
III)
4
≥2L atezo vs doc (n=850)
Histology
Squamous
Non-squamous
All comers
All comers
PD-L1
selected
No
No
Yes (TPS ≥1%)
No
Efficacy by
PD-L1
status
PD-L1
assay
28-8 (Dako) on TCs
22C3 (Dako) on TCs SP142 (Ventana) on ICs and
TCs
0.53
0.76
HR
1–49% (n=591)
≥50% (n=442)
Subgroup
(pooled doses)
doc
HR
pembro
0.1
1
2
0.5
0.2
0.67
ITT (n=1,033)
≥5% (n=81)
<5% (n=144)
≥10% (n=69)
<10% (n=156)
≥1% (n=119)
<1% (n=106)
ITT (
n=272
)
0.69
0.58
0.53
0.70
0.50
0.70
0.39
0.59
HR
Subgroup
doc
HR
nivo
0.1
1 2
0.5
0.2
NQ (n=47)
≥5% (n=181)
<5% (n=274)
≥10% (n=165)
<10% (n=290)
≥1% (n=246)
<1% (n=209)
ITT (n=582)
0.58
0.87
0.43
0.96
0.40
0.96
0.72
HR
Subgroup
doc
HR
nivo
0.1
1
2
0.5
0.2
doc
atezo
HR
TC1/2/3 or IC1/2/3 (n=463)
TC0 and IC0 (n=379)
TC3 or IC3 (n=137)
TC2/3 or IC2/3 (n=265)
ITT (n=850)
0.73
0.41
0.67
0.74
0.75
HR
Subgroup*
<1% not available
study design
2
1
0.1
0.2
0.5









