
Sorafenib
Safety Profile
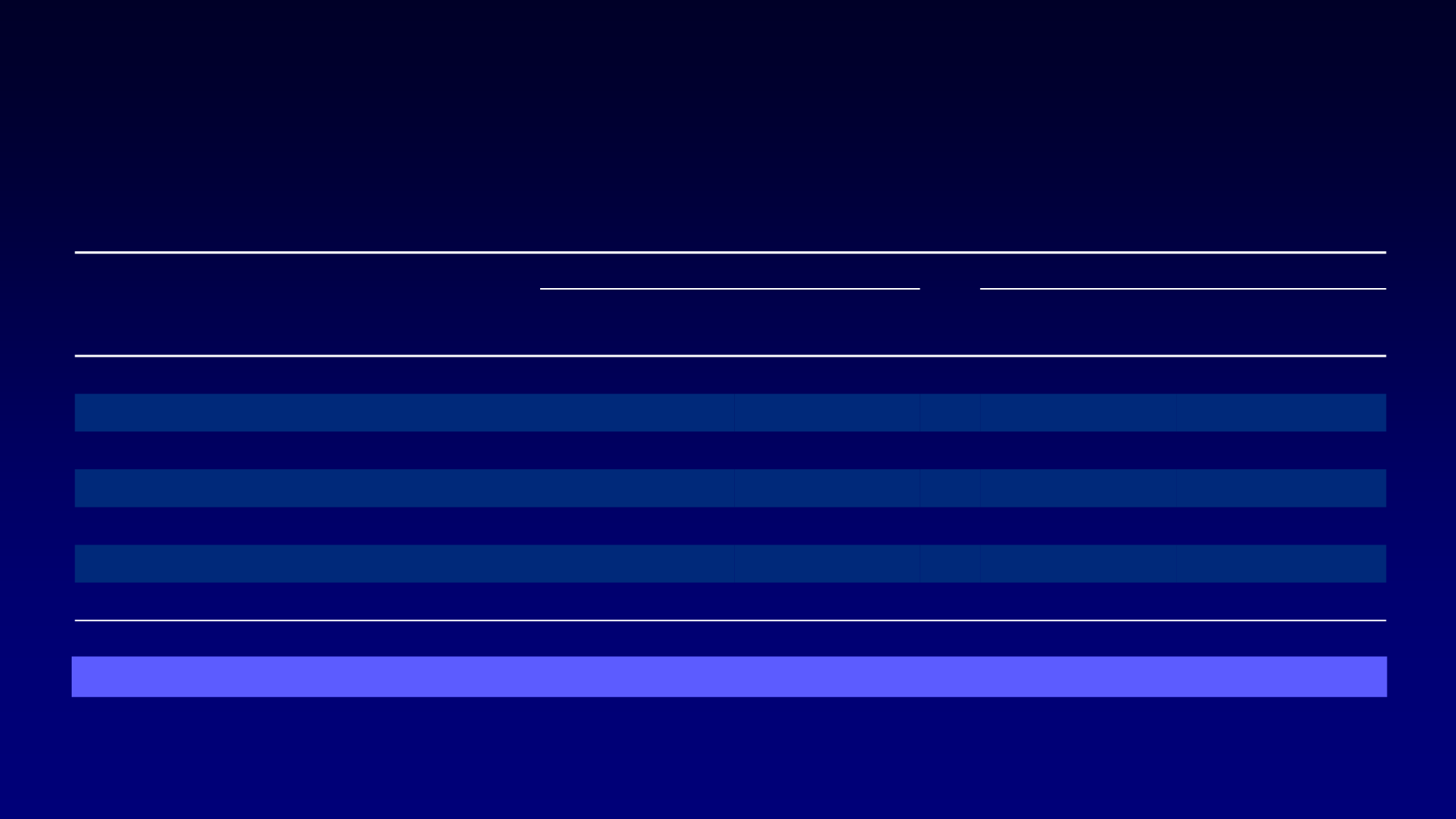
Most Common Treatment-Emergent AEs (Double-blind Period)
Safety results are consistent with the known safety profile of sorafenib.
2
AE,
1
Sorafenib (n = 207)
Placebo (n = 209)
Any Grade
Grade 3/4
Any Grade
Grade 3/4
HFSR
a
76.3%
20.3%
9.6%
0%
Diarrhea
68.6%
5.8%
15.3%
1.0%
Alopecia
67.1%
0%
7.7%
0%
Rash/desquamation
50.2%
4.8%
11.5%
0%
Fatigue
49.8%
5.8%
25.4%
1.4%
Weight loss
46.9%
5.8%
13.9%
1.0%
Hypertension
40.6%
9.7%
12.4%
2.4%
AE, adverse event; HFSR, hand-foot skin reaction; MedDRA, Medical Dictionary for Regulatory Activities.
a
HFSR cases were usually grade 1 or 2 and generally appeared during the first 6 weeks of treatment.
1. National Cancer Institute. Common Terminology Criteria for Adverse Events (NCI CTCAE). Version 3.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.Accessed April 21,
2014. 2. Brose MS, et al.
Lancet.
2014;384(9940): 319-328.









