
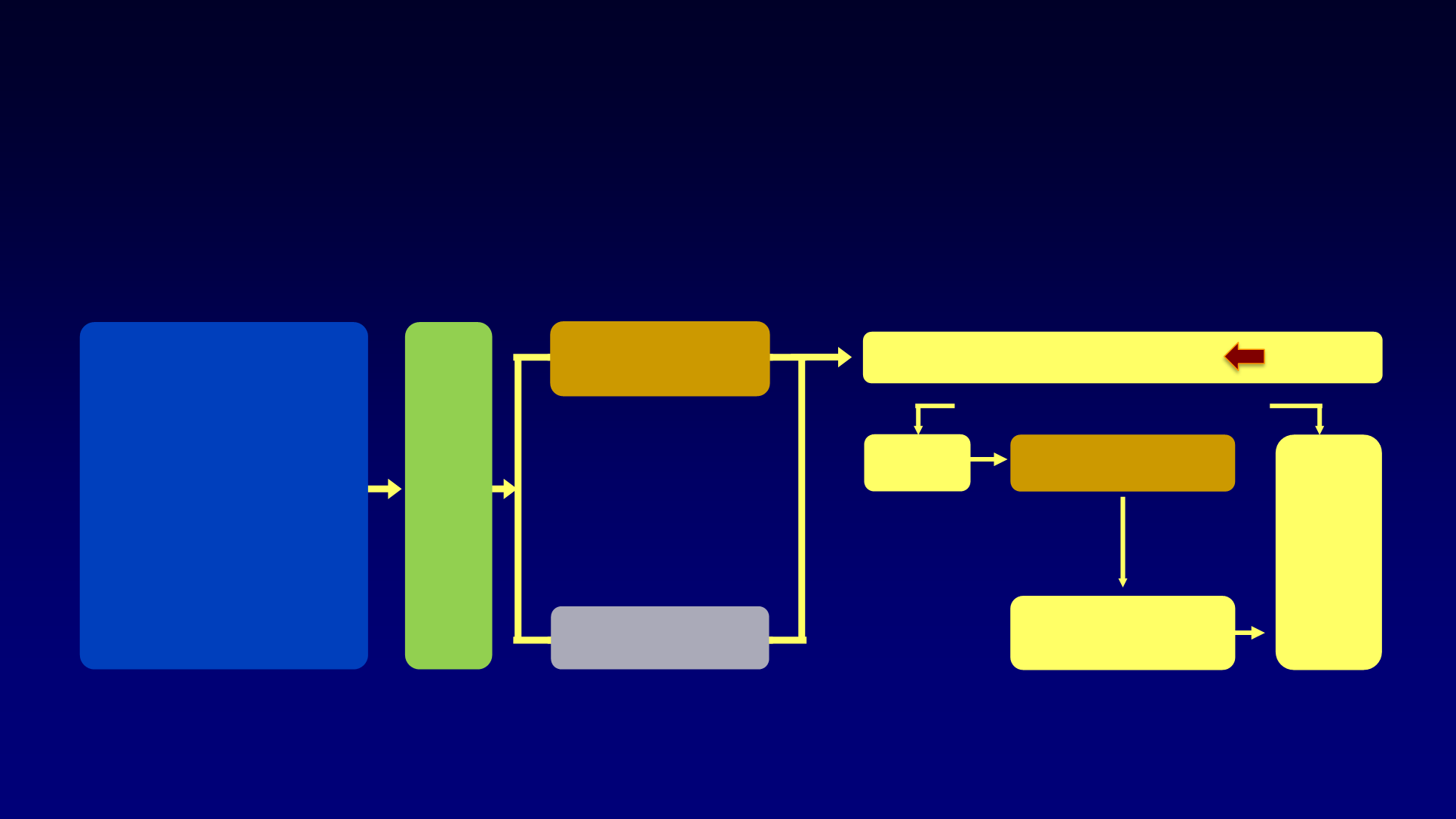
DECISION Trial:
Sorafenib Versus Placebo in Locally Advanced/Metastatic RAI-Refractory DTC
•
An international, multicenter, randomized, double-blind, phase 3 study of sorafenib versus placebo in patients with
locally advanced/metastatic RAI-refractory DTC
1,2
Placebo
Sorafenib
400 mg PO BID
PFS
(primary endpoint)
Investigator’s decision
Off
study
Unblind
Sorafenib
(crossover or continue)
Follow-up until disease
progression
Patients
a
• Locally advanced or
metastatic DTC
• Progression within
14 months
• RAI-refractory
• ≥ 1 measurable lesion
• No prior anticancer treatment
• ECOG PS ≤ 2
Randomization (1:1)
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; PO, by mouth.
a
Additional patient eligibility criteria exist.
1. ClinicalTrials.gov identifier # NCT00984282.
https://clinicaltrials.gov/ct2/show/NCT00984282.2. Brose MS, et al.
Lancet.
2014;384(9940):319-328.









