
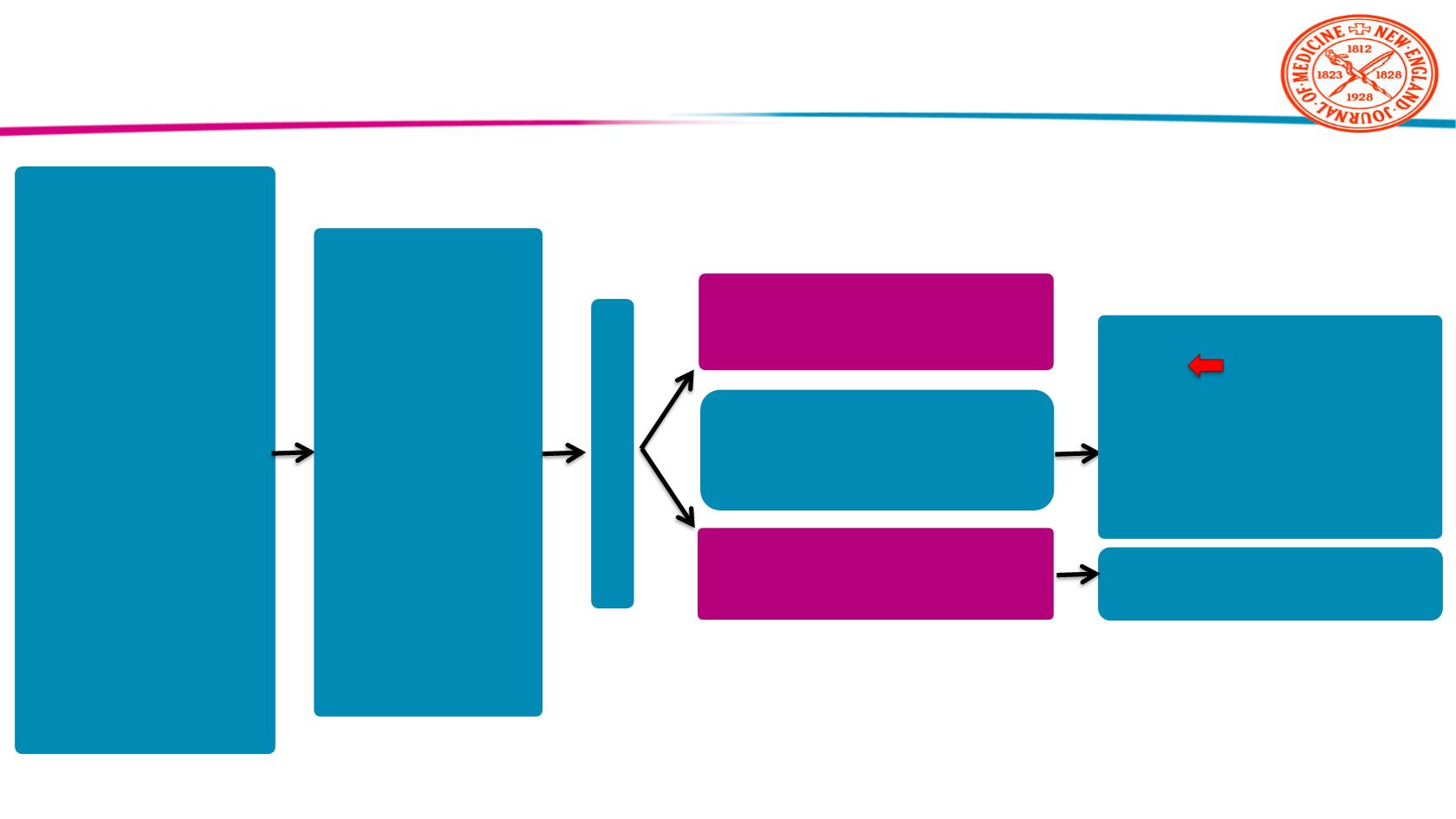
16
Patients with
DTC (n=392)
•
≥18 years old
•
IRR evidence of
progression within
previous 13 months
•
131
I-refractory disease
•
Blood pressure
≤150/90 mmHg
•
Up to 1 prior VEGF or
VEGFR-targeted
therapy
Placebo (n=131)
Daily PO
Lenvatinib (n=261)
24 mg daily PO
Stratification
•
Geographic region
(Europe,
N. America, Other)
•
Prior VEGF/
VEGFR targeted
therapy
(0,1)
•
Age
(≤65 years,
>65 years)
Treatment until
IRR-verified disease
progression by RECIST 1.1
Lenvatinib
(Optional, open-label)
Randomization 2:1
Global, randomized, double-blind Phase III trial
DTC, differentiated thyroid cancer;
131
I, radioiodine; IRR, independent radiologic review, ORR, overall response rate; OS,
overall survival; PFS, progression-free survival; PO, by mouth; VEGF, vascular endothelial growth factor; VEGFR, vascular
endothelial growth factor receptor
Primary endpoint
●
PFS
Secondary endpoints
●
ORR
●
OS
●
Safety
SELECT Trial
Schlumberger M, et al. N Engl J Med 2015;372:621-30. DOI: 10.1056/NEJMoa1406470









