
11
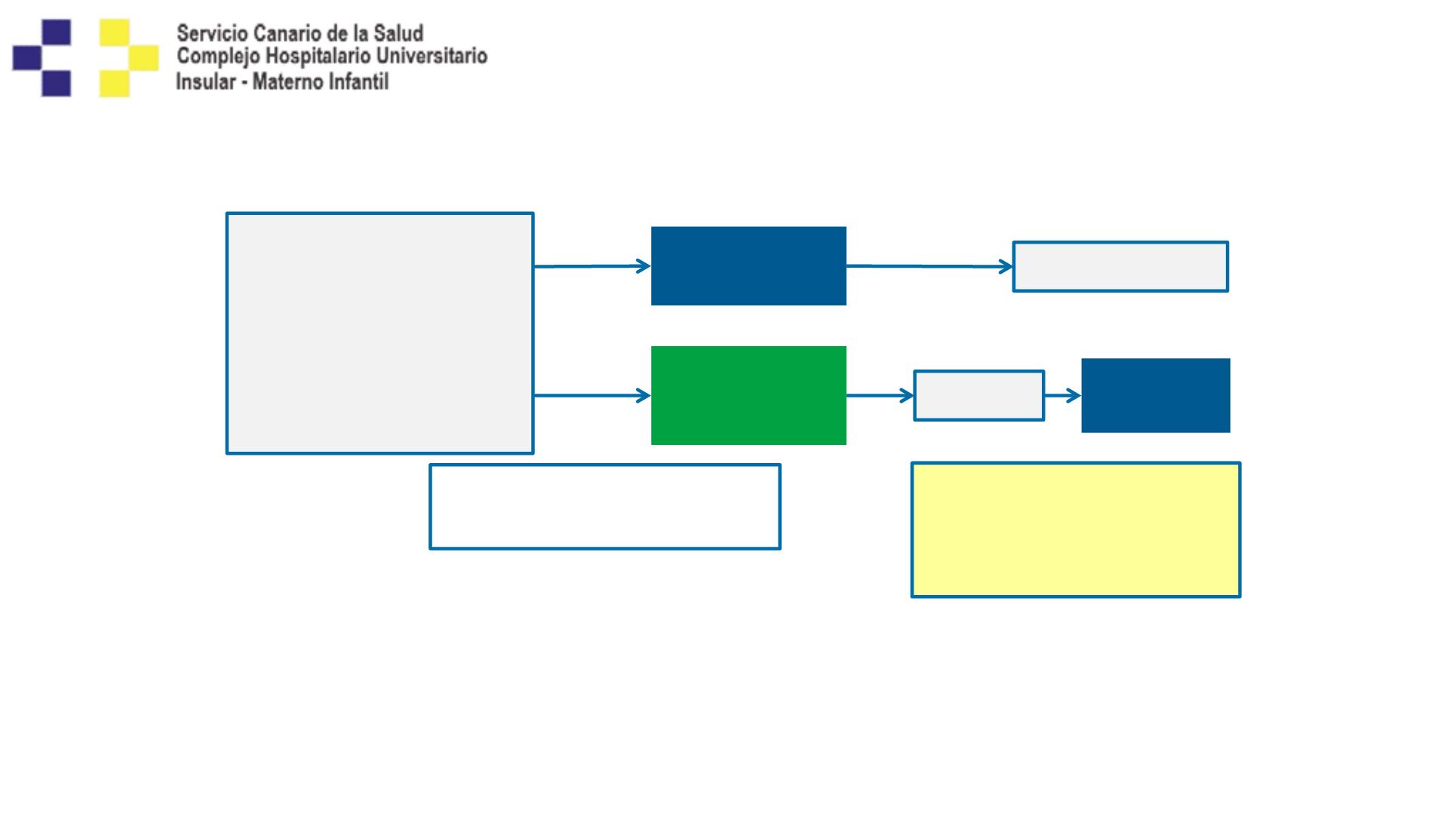
Phase 3 CheckMate 026 Study Design:
Nivolumab vs Chemotherapy in First-line NSCLC
Primary endpoint:
PFS (≥5% PD-L1+)
d
Secondary endpoints:
• PFS (≥1% PD-L1+)
d
• OS
• ORR
d
Nivolumab
3 mg/kg IV Q2W
n = 271
Randomize 1:1
Key eligibility criteria:
• Stage IV or recurrent NSCLC
• No prior systemic therapy for
advanced disease
• No
EGFR/ALK
mutations sensitive to
available targeted inhibitor therapy
• ≥1% PD-L1 expression
a
• CNS metastases permitted if
adequately treated at least 2 weeks
prior to randomization
Chemotherapy
(histology dependent)
b
Maximum of 6 cycles
n = 270
Disease progression or
unacceptable toxicity
Disease
progression
Crossover
nivolumab
c
(optional)
Tumor scans Q6W until
wk 48 then Q12W
a
Dako 28-8 validated; archival tumor samples obtained ≤6 months before enrollment were permitted; PD-L1 testing was centralized
b
Squamous: gemcitabine 1250 mg/m
2
+ cisplatin 75 mg/m
2
; gemcitabine 1000 mg/m
2
+ carboplatin AUC 5; paclitaxel 200 mg/m
2
+ carboplatin AUC 6;
Non-squamous: pemetrexed 500 mg/m
2
+ cisplatin 75 mg/m
2
; pemetrexed 500 mg/m
2
+ carboplatin AUC 6; option for pemetrexed maintenance therapy
c
Permitted if crossover eligibility criteria met, including progression confirmed by independent radiology review
d
Tumor response assessment for PFS and ORR per RECIST v1.1 as determined by independent central review
Stratification factors at randomization:
• PD-L1 expression (<5% vs ≥5%)
a
• Histology (squamous vs non-squamous)
ESMO 2016
Socinski et al ESMO 2016









