
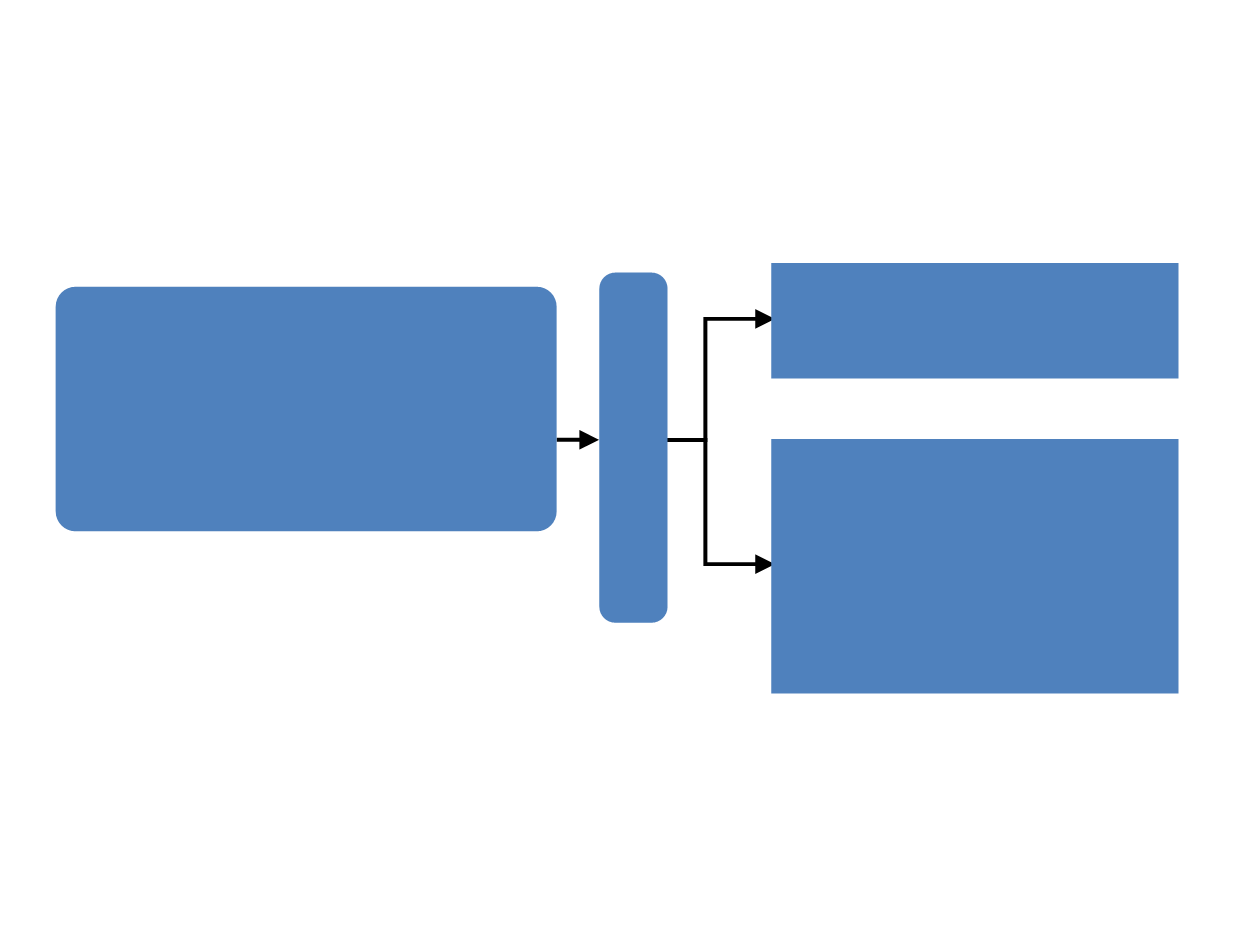
TREnd study design
•
Post-menopausal ER+/HER2– mBC
•
Pre-treated with 1 or 2 lines of ET for
mBC
•
1 prior line of CHT for mBC permitted
•
FFPE tumor available for biomarkers
Palbociclib
125 mg/d
3w on/1w off
+
same ET
as pre-progression
(Aromatase Inhibitor or Fulvestrant)
1:1
Palbociclib
125 mg/d
3w on/1w off
Stratification Factors:
Disease site (visceral vs other)
number of prior line of ET for mBC (1 vs. 2)
duration of prior line of ET for mBC (>6 vs. ≤6 months);
Key eligibility criteria:
R
A
N
D
O
M
I
Z
A
T
I
O
N
N = 115
Malorni L, et al. ASCO 2017









