
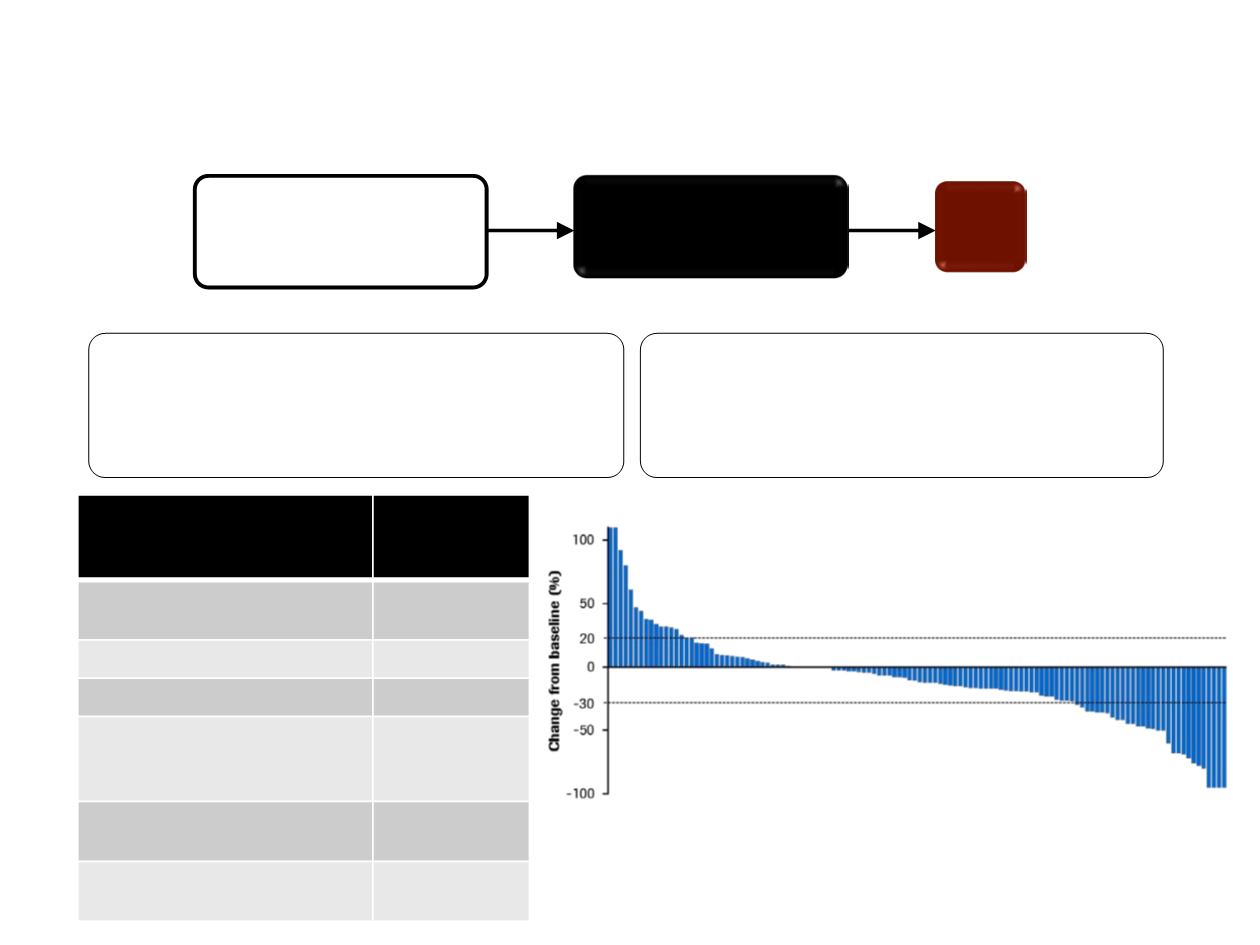
MONARCH 1 (Phase II): Abemaciclib monotherapy in HR+/
HER- BC, after chemotherapy for advanced disease
2L/3L HR+/HER2– mBC
(inc. prior taxane)
N = 132
Abemaciclib 200 mg BID
D1–28
PD
Primary endpoint:
•
Objective response rate (investigator-
assessed)
Key secondary endpoints:
•
Overall survival
•
Duration of response
•
Progression-free survival
Dickler MN, et al. Submitted
Abemaciclib
200 mg
N = 132
Confirmed ORR, %
(95% CI)
19.7
(13.3, 27.5)
Clinical benefit rate, %*
42.4
Median time to response, mo
3.7
Median DoR, mo
6-mo DoR, %
12-mo DoR, %
8.6
70.4
28.2
Median PFS, mo
(95% CI)
6.0
(4.2, 7.5)
Median OS, mo
(95% CI)
17.7
(16.0, NR)
Disease Control Rate (CR + PR + SD) = 67.4%
Progressive disease (n = 34)
Stable disease (n = 63)
Partial response (n = 26)
Not assessed (n = 9)









