
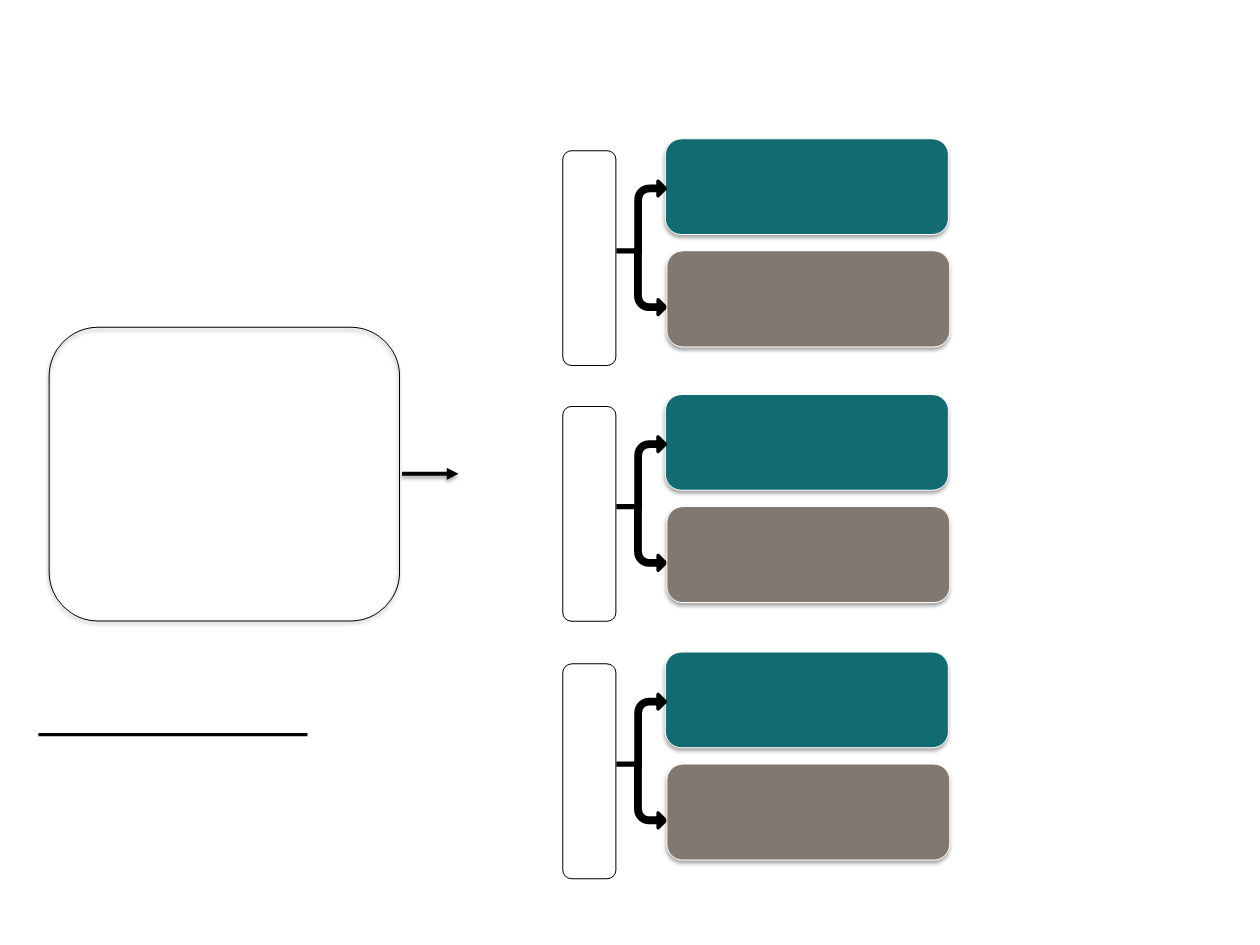
Endocrine resistance CDK inhibitors Study Designs
•
HR+, HER2- ABC
•
Pre/peri & Postmenopausal*
•
Progressed on prior
endocrine therapy:
–
On or within 12 mo
adjuvant
–
On therapy for ABC
abemaciclib: 150 mg
BID
Abemaciclib
plus
Fulvestrant
Randomization
2 :1
placebo
plus
Fulvestrant
N=669
abemaciclib: 150 mg
BID
Palbociclib
plus
Fulvestrant
Randomization
2 :1
placebo
plus
Fulvestrant
N=521
abemaciclib: 150 mg
BID
Ribociclib
plus
Fulvestrant
Randomization
1 :1
placebo
plus
Fulvestrant
PALOMA-3
1
MONALEESA-3
2
*
(no data yet)
MONARCH-2
3
Primary endpoint:
Investigator-assessed PFS
*Only postmenopausal
1
Turner NC, et al. N Engl J Med 2015;
2
Not reported;
3
Sledge G, et al. J Clin Oncol 2017









