
First-line CDK inhibitors Study Design in pre/peri-
menopausal-only patients
•
HR+, HER2- ABC
•
Pre-peri menopausal
•
No prior ET
•
≤ 1 line of chemo for ABC
abemaciclib: 150 mg
BID
Ribociclib
plus
Tamoxifen/NSAI + goserelin
Randomization
1 :1
placebo
plus
Tamoxifen/NSAI + goserelin
N=672
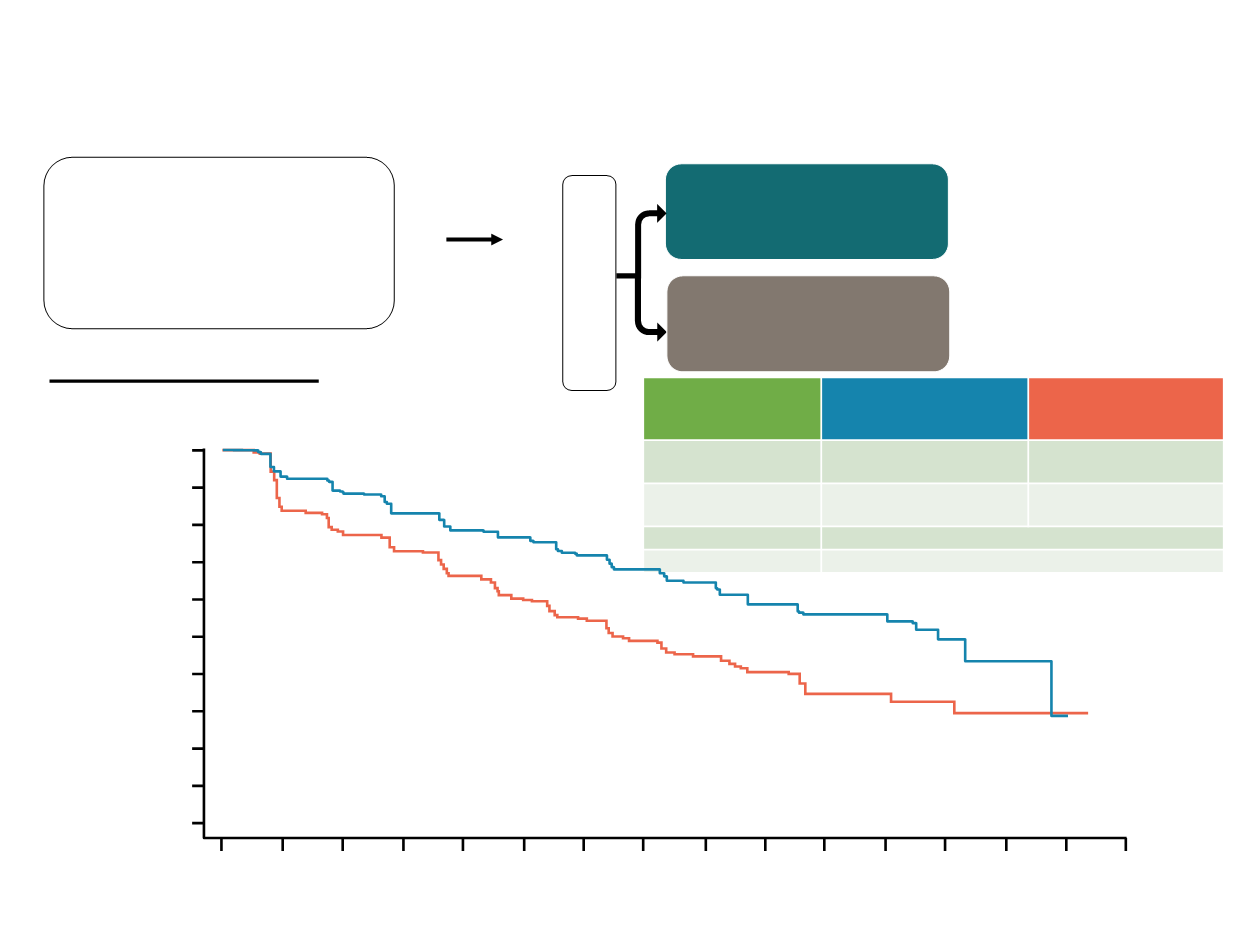
MONALEESA-7
Primary endpoint:
Investigator-assessed PFS
Tripathy D, et al. SABCS 2017
Probability of PFS (%)
Time (months)
PFS (investigator
assessment)
Ribociclib +
tamoxifen/NSAI n=335
Placebo +
tamoxifen/NSAI
n=337
Number of events, n
(%)
131 (39.1)
187 (55.5)
Median PFS, months
(95% CI)
23.8
(19.2–NR)
13.0
(11.0–16.4)
Hazard ratio (95% CI)
0.553 (0.441–0.694)
One-sided p value
0.0000000983
10
8
6
4
2
0
100
80
60
40
20
0
30
28
26
24
22
20
18
16
14
12
10
30
50
70
90









