
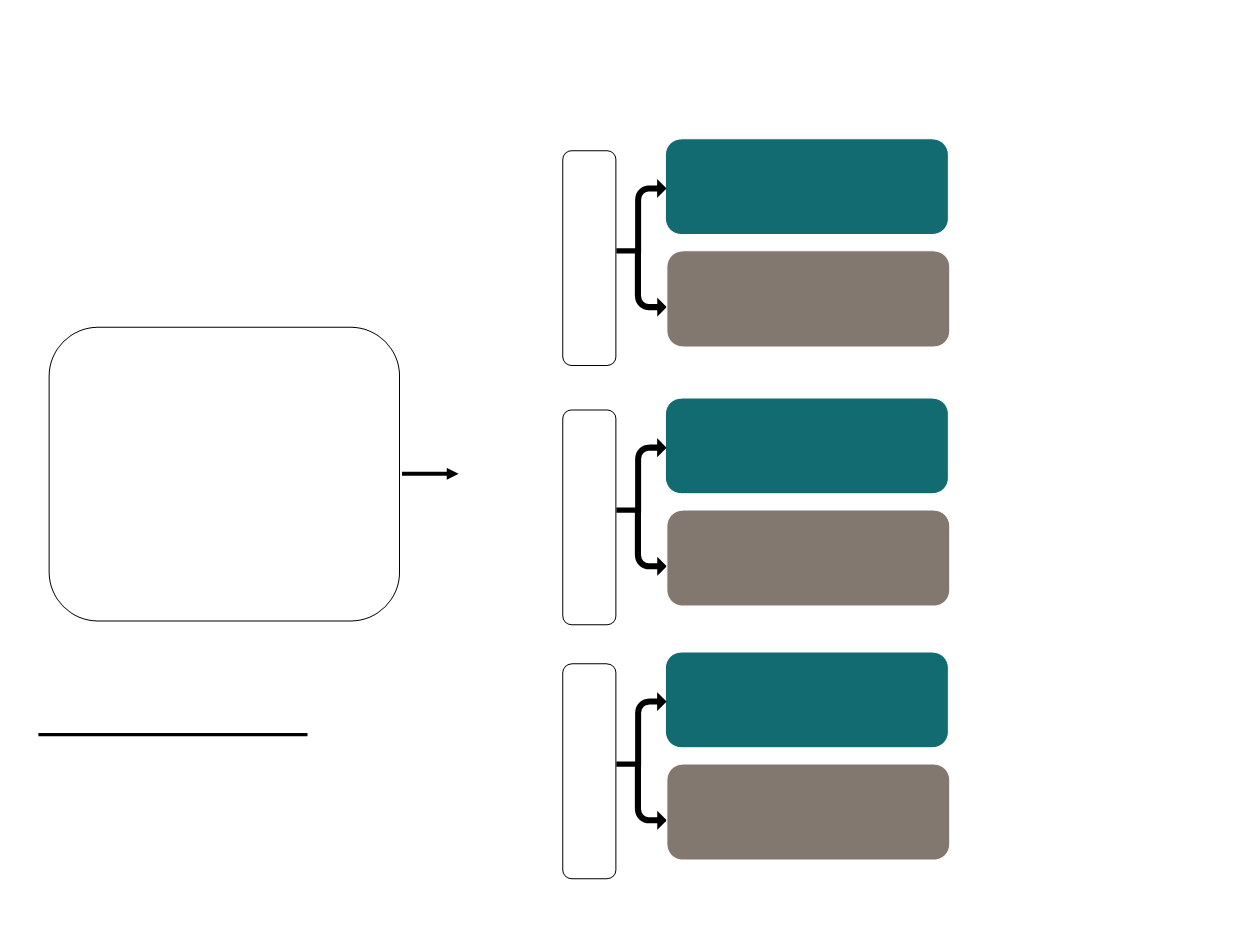
First-line CDK inhibitors Study Designs
•
HR+, HER2- ABC
•
Postmenopausal
•
Noprior systemic therapy in
this setting
•
If neoadjuvant or adjuvant ET
administered, a disease free
interval of >12 months since
completion of ET
•
ECOG PS ≤1
abemaciclib: 150 mg
BID
Abemaciclib
plus
AI
Randomization
2 :1
placebo
plus
AI
N=493
abemaciclib: 150 mg
BID
Palbociclib
plus
AI
Randomization
2 :1
placebo
plus
AI
N=666
abemaciclib: 150 mg
BID
Ribociclib
plus
AI
Randomization
1 :1
placebo
plus
AI
N=668
PALOMA-2
1
MONALEESA-2
2
MONARCH-3
3
Primary endpoint:
Investigator-assessed PFS
1
Finn RS, et al. N Engl J Med 2016;
2
Hortobagyi G, et al. N Engl J Med 2016;
3
di Leo A, et al. J Clin Oncol 2017









