
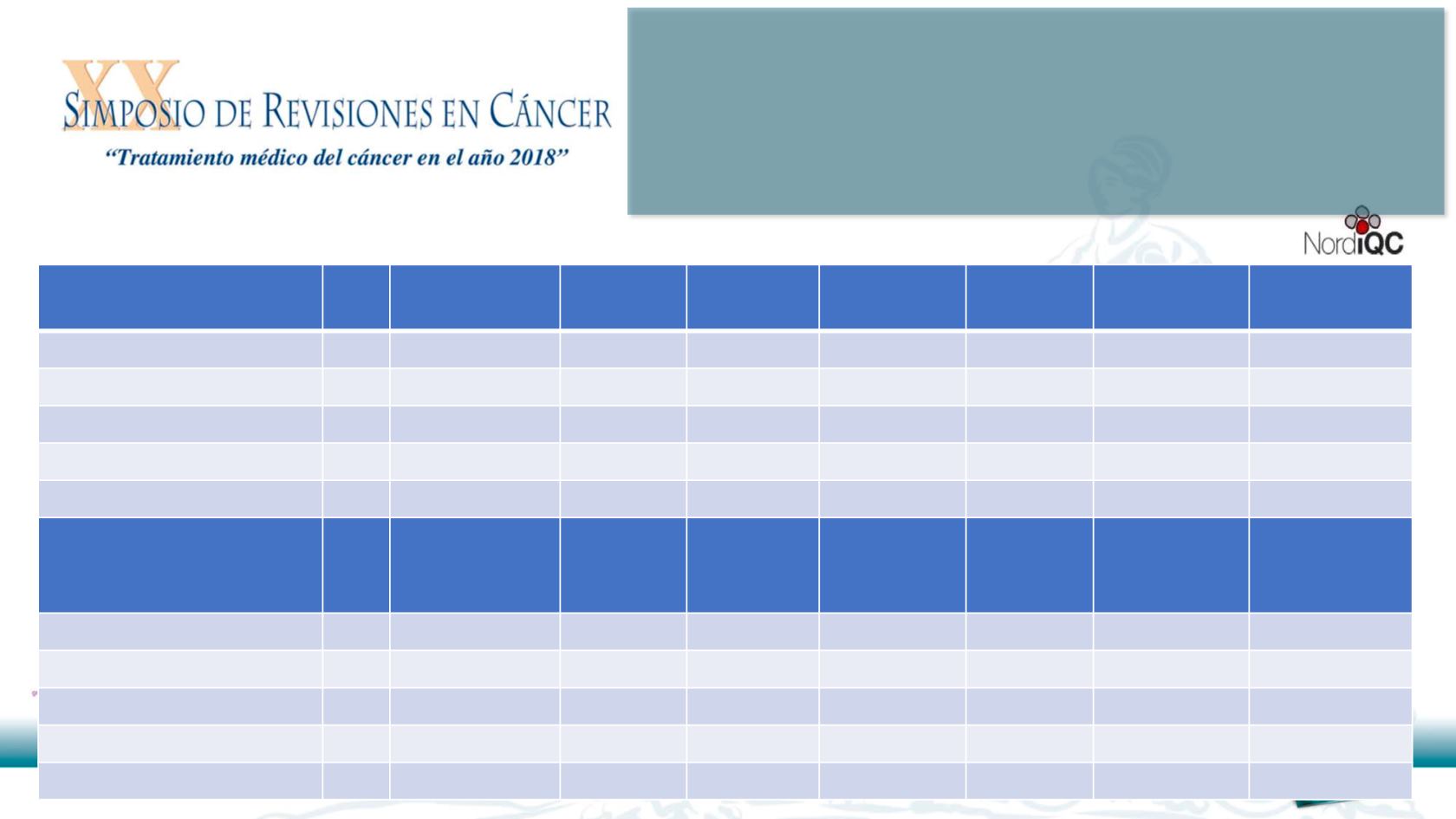
Table 1.
Assessment marks for IHC assays and antibodies run C1, PD-L1 IHC
CE-IVD / FDA approved
PD-L1 assays
n
Vendor
Optimal
Good
Borderline
Poor
Suff.
1
Suff. OPS
2
22C3 pharmDX, SK006
12
Dako/Agilent
10
1
0
1
92%
92%
22C3 pharmDX, SK006
4
2
Dako/Agilent
0
0
1
1
-
-
28-8 pharmDX, SK005
7
Dako/Agilent
3
3
1
0
86%
86%
SP263, 790-4905
16
Ventana/Roche
9
2
2
3
69%
77%
SP142, 740-4859
1
Ventana/Roche
0
0
0
1
-
-
Antibodies
3
for laboratory
developed PD-L1 assays,
conc. antibody
n
Vendor
Optimal
Good
Borderline
Poor
Suff.
1
Suff. OPS2
mAb clone 22C3
13
Dako/Agilent
1
1
4
7
15%
-
mAb clone E1L3N
8
Cell Signaling
1
1
1
5
25%
-
mAb CAL10
1
Biocare
0
0
1
0
-
-
rmAb clone 28-8
6
Abcam
0
1
1
4
17%
-
rmAb clone ZR3
1
Zeta Corporation
1
0
0
0
-
-
PD-L1 standardization provides
precision in assay results









