
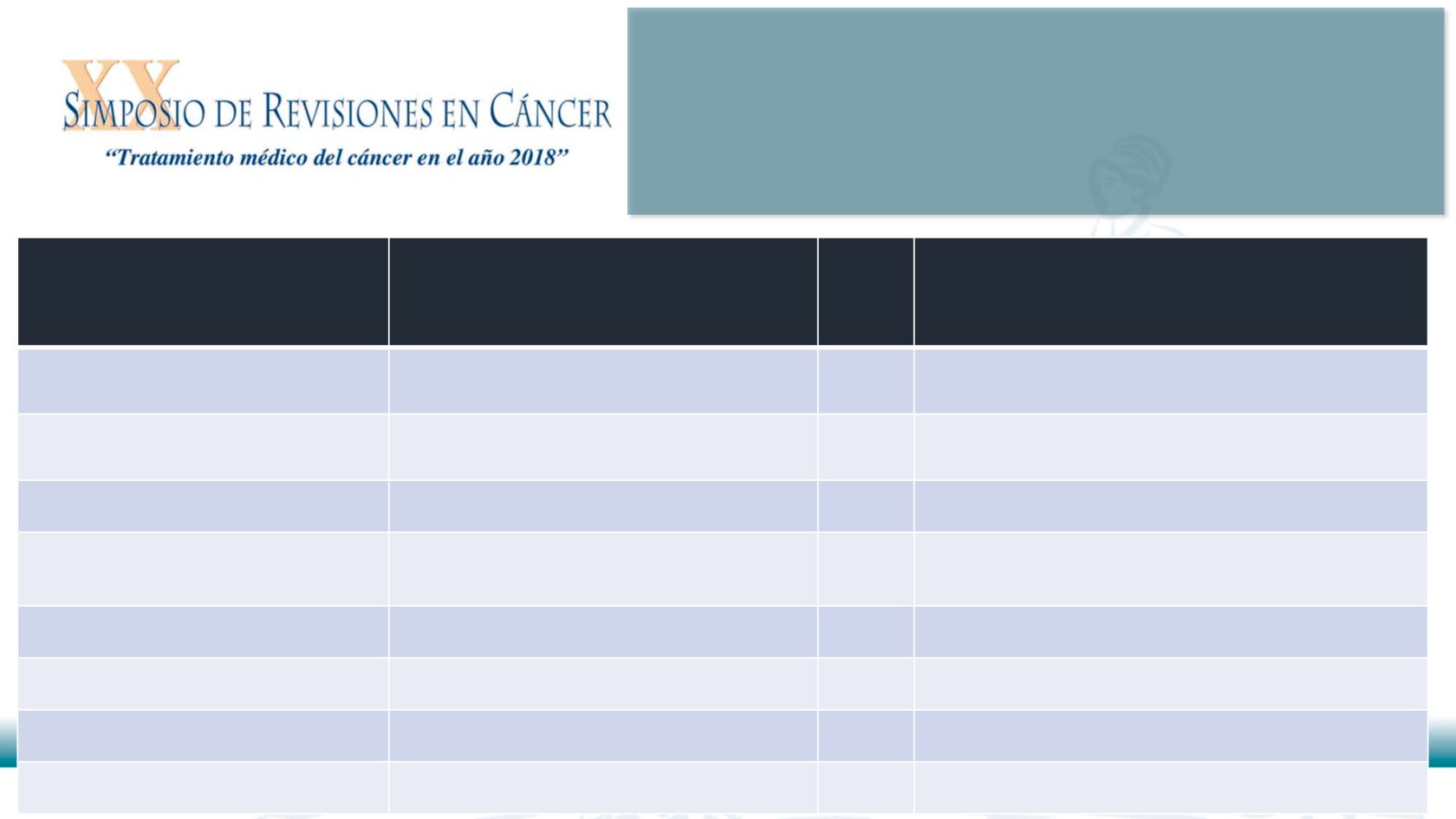
Reference
Antibodies
N Concordance of LDT vs FDA-
approved assay
Neuman, T et al. J Thorac Oncol
2016
22C3 on 3 platforms
41
85%
Cogswell, J et al. Mol Diag Ther
2017
28-8, E1L3N
20
28-8 more sensitive
Scheel, AH et al. Mod Pathol 2016
SP142, SP263, 28-8, 22C3, E1L3N
30
55% of concordance
Scheel, AH et al. Histopathology
2018
SP142, SP263, 28-8, 22C3, E1L3N ,
Q1R (11 different LDT)
21
42% of concordance
Smith, J et al. Diag Pathol 2016
SP263, E1L3N
100
SP263 more sensitive than E1L3N
Rimm, DL et al. JAMA Oncol 2017
SP142, 28-8, 22C3, E1L3N
90
E1L3N similar to 28-8 and higher than 22C3
Adam, J et al. Ann Oncol 2018
SP263, 28-8, 22C3, E1L3N
41
51.8% of concordance
Conde, E, et al. Histopathol 2017
SP263, SP142, E1L3N
40
Similar
Eight key publications for PD-L1
LDT performance in NSCLC









