
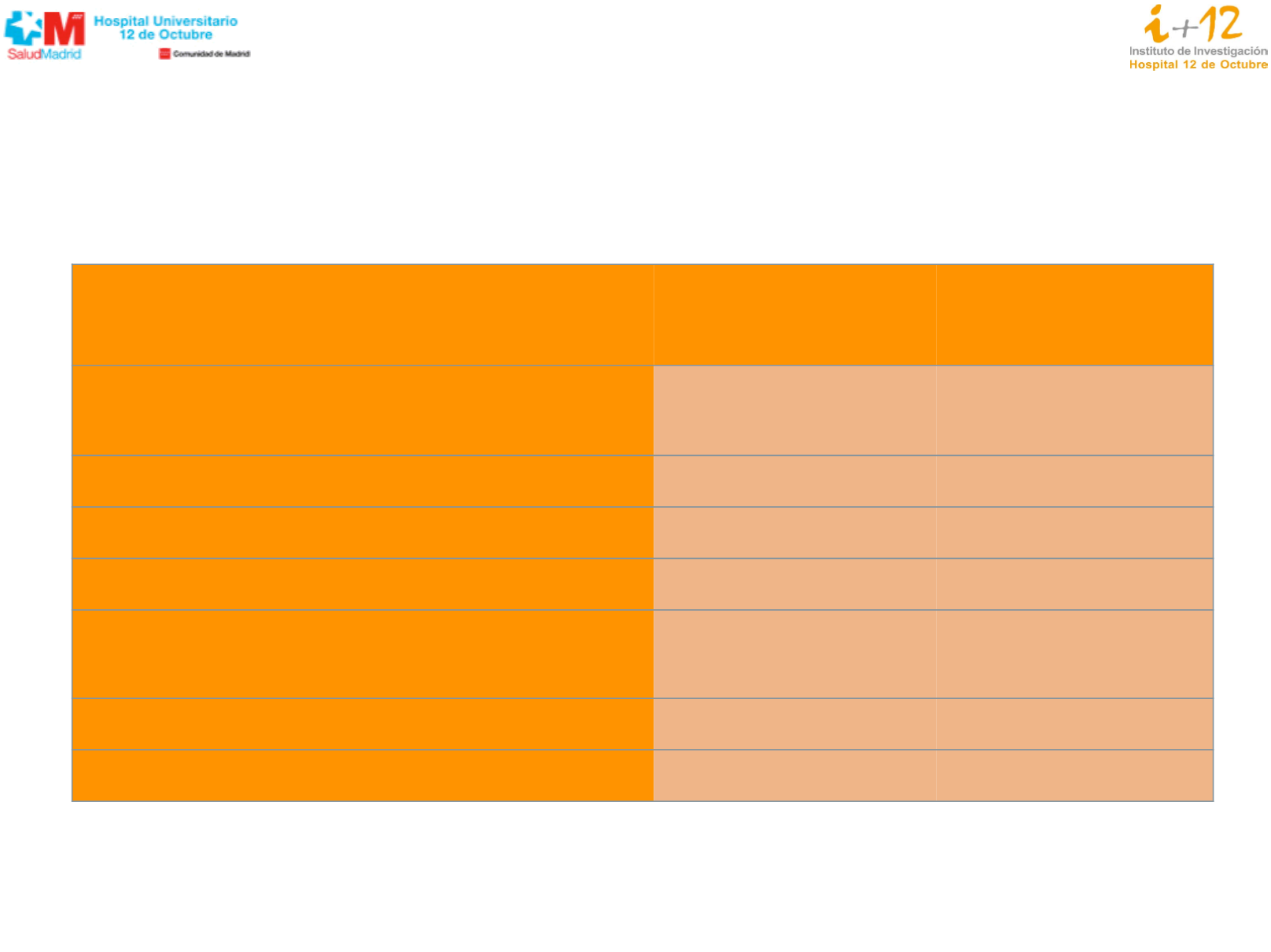
Shaw, et al. WCLC 2015; Ou, et al. ASCO 2015;
Alectinib
(n=70)
Chemotherapy
(n=34)
All Grade Adverse Events (AEs)
54 (77.1%)
Serious AEs
13 (18.6%)
5 (14.7%)
Grade 3-5 AEs
19 (27.1%)
14 (41.2%)
Fatal AEs
0 (0%)
1 (2.9%)
AEs leading to treatment discontinuation
4 (5.7%)
3 (8.8%)
AEs leading to dose reduction
3 (4.3%)
4 (11.8%)
AEs leading to dose interruption
13 (18.6%)
3 (8.8%)
ALUR: phase III trial of alectinib versus
chemotherapy in previously treated ALK+ NSCLC









