
S.Novello ESMO 2017
ALUR: phase III trial of alectinib versus
chemotherapy in previously treated ALK+ NSCLC
Alectinib Arm (N=72)
Chemotherapy Arm
(N=35)
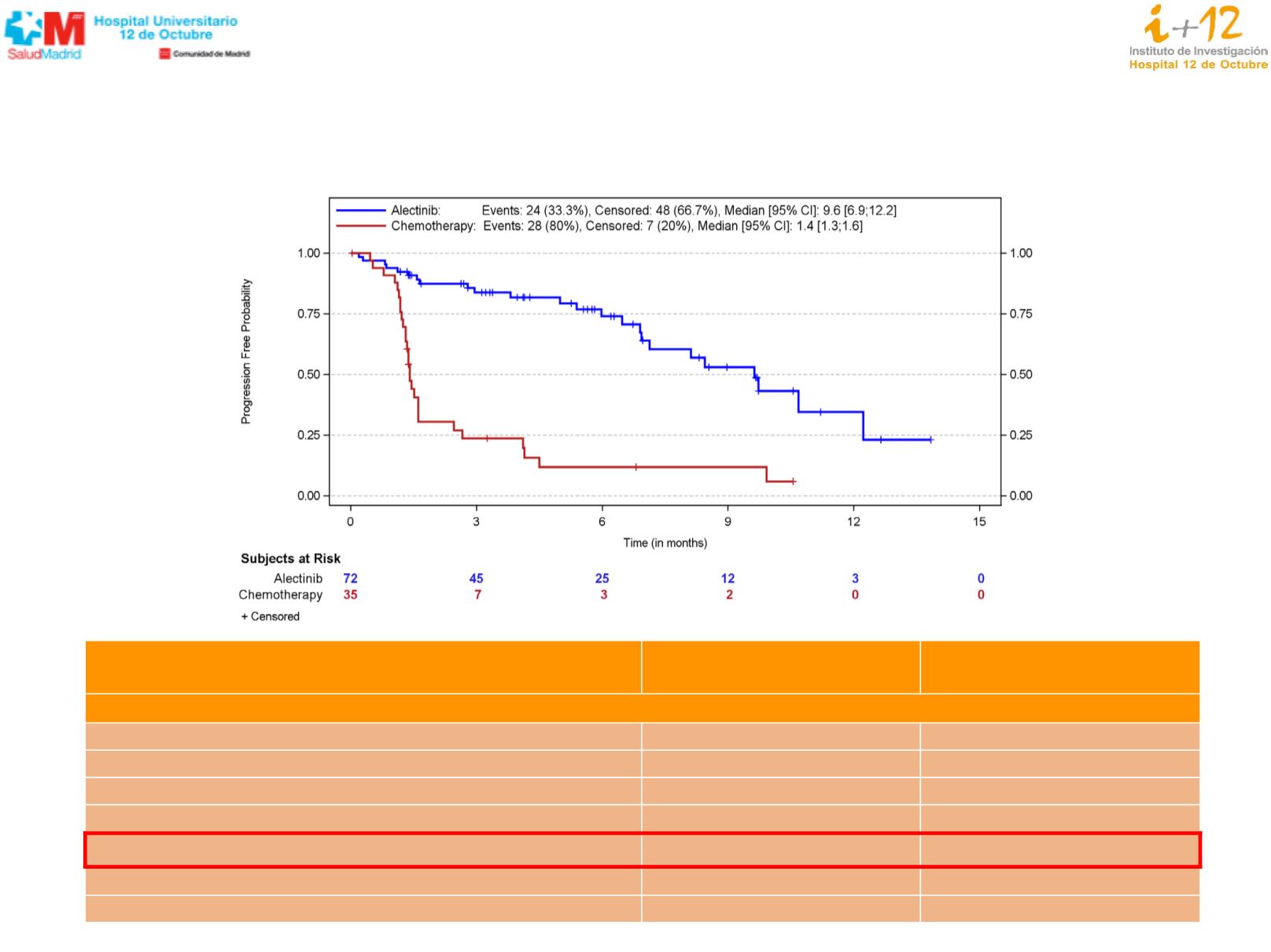
PFS (investigator) – ITT Population
1
KM estimates of PFS (in months)
Events, n (%)
24 (33.3)
28 (80.0)
Censored, n (%)
48 (66.7)
7 (20.0)
Median (95% CI
2
)
9.6 (6.9, 12.2)
1.4 (1.3, 1.6)
Hazard ratio (alectinib vs. chemotherapy)
3
0.15
95% CI
0.08, 0.29
Log-rank test p-value
4
<0.001









