
*Progression events that did not occur within 2 scheduled visits (plus visit window) of the last evaluable assessment (or randomisation) were censored and therefore excluded in the number of events;
#
A p-value of <0.0015 was required for
statistical significance at current maturity
CI, confidence interval; CNS, central nervous system; HR, hazard ratio; NC, not calculable; PFS, progression-free survival; SoC, standard-of-care
FLAURA data cut-off: 12 June 2017
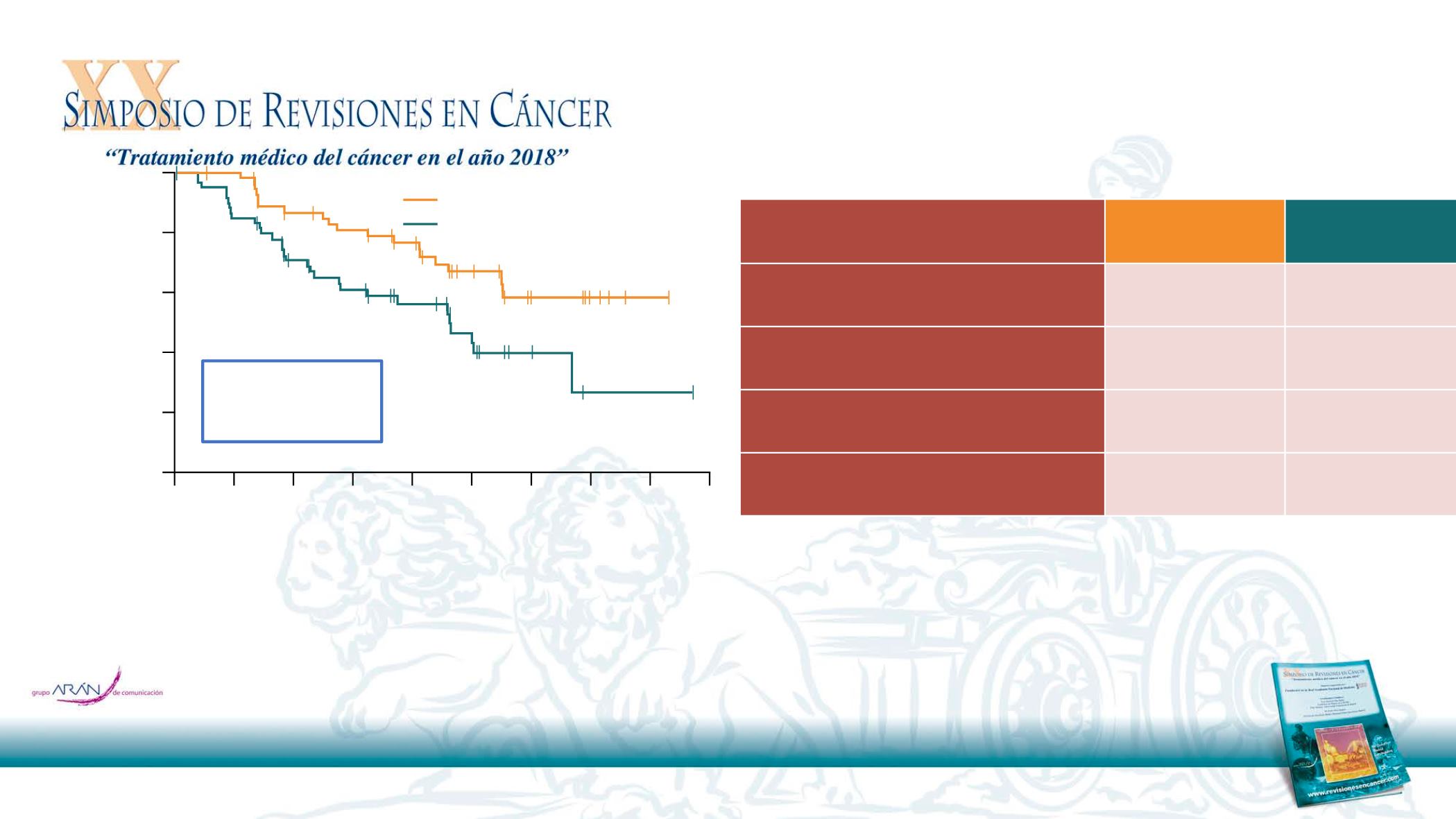
Median CNS PFS, months (95% CI
)
NC (16.5, NC)
13.9
(8.3, NC)
HR 0.48
(95% CI 0.26, 0.86)
p=0.014
Osimertinib
(n=61)
SoC
(n=67)
Median follow-up for CNS PFS, months
12.4
7.0
Total number of events (CNS progression
or death), %
30
45
Pts with CNS progression other than
death, %*
20
39
Progression in new CNS lesions, %
12
30
CNS PFS was nominally statistically significant
•
CNS PFS analysis was third in the hierarchical statistical testing strategy and, as OS did not reach formal statistical significance (HR 0.63 [95% CI 0.45, 0.88]; p=0.0068),
#
CNS
PFS could not be formally tested for statistical significance
Osimertinib (N=61)
SoC (N=67)
0.2
0.4
0.6
0.8
1.0
0.0
0
3
6
9
12
15
18
21
24
27
Time from randomisation (months)
Probability of progression-free survival
No. at risk
Osimertinib
SoC
Presented by J Vansteenkiste at ESMO Asia 2017, 17–19 November 2017, Singapore
Proferred Paper Session 1, Abstract LBA5. Ann Oncol 2017;28 (suppl_10): mdx729.007









