
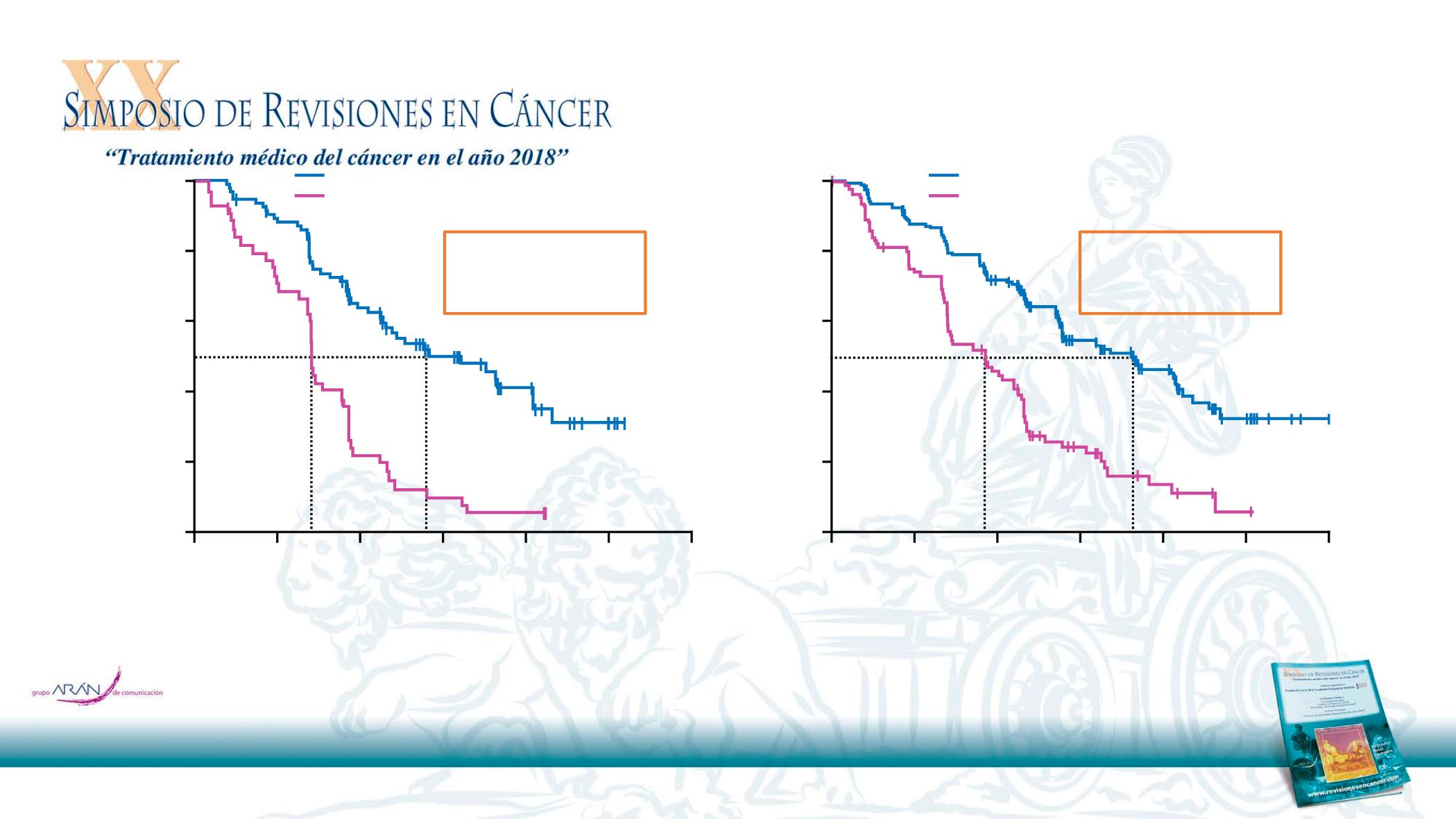
PFS benefit in AURA3 patients with CNS metastases at baseline
With CNS metastases
Without CNS metastases
Population: intent-to-treat
Progression-free survival defined as time from randomisation until date of objective disease progression or death. Progression included deaths in the absence of RECIST progression.
Tick marks indicate censored data. CNS metastases determined programmatically from baseline data of CNS lesion site, medical history, and/or surgery, and/or radiotherapy.
Probability of
progression-free survival
1.0
0.8
0.6
0.4
0.2
0
No. at risk
Osimertinib
Platinum-pemetrexed
0
3
6
9
12
15
18
93
51
80
32
46
9
27
4
14
2
4
0
0
0
Months
Osimertinib (n=93)
Platinum-pemetrexed (n=51)
Median PFS, months (95% CI)
8.5 (6.8, 12.3)
4.2 (4.1, 5.4)
HR 0.32
(95% CI 0.21, 0.49)
Probability of
progression-free survival
1.0
0.8
0.6
0.4
0.2
0
0
3
6
9
12
15
18
186
89
160
61
116
35
61
13
36
5
9
1
0
0
Months
Osimertinib (n=186)
Platinum-pemetrexed (n=89)
Median PFS, months (95% CI)
10.8 (8.3, 12.5)
5.6 (4.2, 6.8)
HR 0.40
(95% CI 0.29, 0.55)
Mok et al. NEJM 2017









