
Inst itut Català d'Oncologia
Institut Català d’Oncol gia
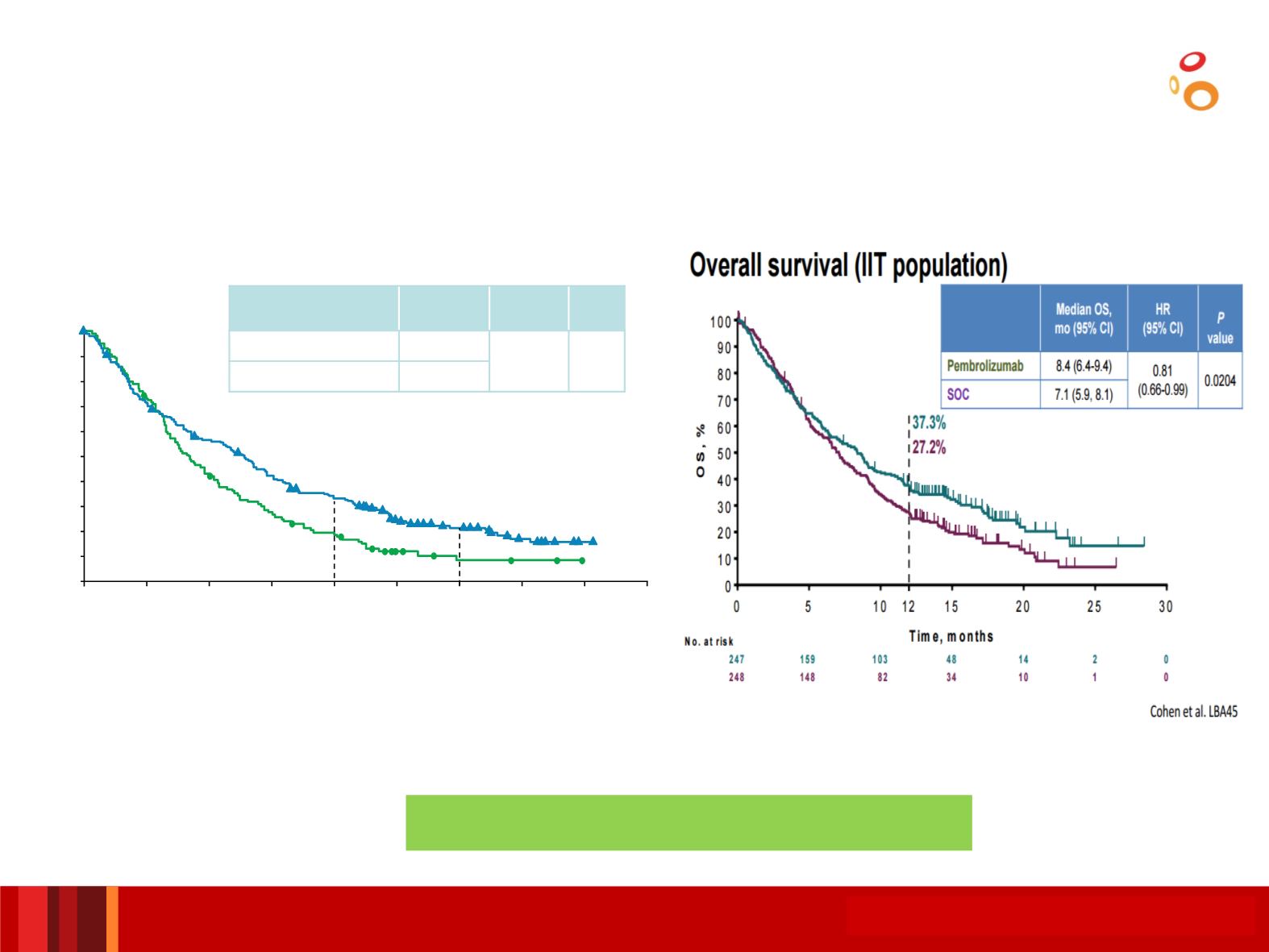
Phase III:
Nivolumab
vs Monotherapy vs
Pembrolizumab
•240
•169
•132
•98
•76
•45
•27
•12
•3
•121
•88
•51
•32
•22
•9
•4
•3
•0
•
Months
•0
•3
•6
•9
•12
•15
•18
•21
•24
•
OS (%)
•0
•10
•20
•30
•40
•50
•60
•70
•80
•100
•90
•
Nivo
•
IC
•
No. of patients at risk
19.7%
•
34.0%
•
21.5%
•
8.3%
Checkmate 141
vs
Keynote 040
Median OS,
mo (95% CI)
HR
(97.73% CI)
p-
value
Nivolumab (n = 240)
7.5 (5.5, 9.1)
0.70
(0.51, 0.96)
0.0101
IC (n = 121)
5.1 (4.0, 6.0)
•Ferris RL, et al.
N Engl J Med
2016;375:1856–1867.
•Cohen E, et al. ESMO 2017
•Independientemente status PDL1









