
Inst itut Català d'Oncologia
Institut Català d’Oncol gia
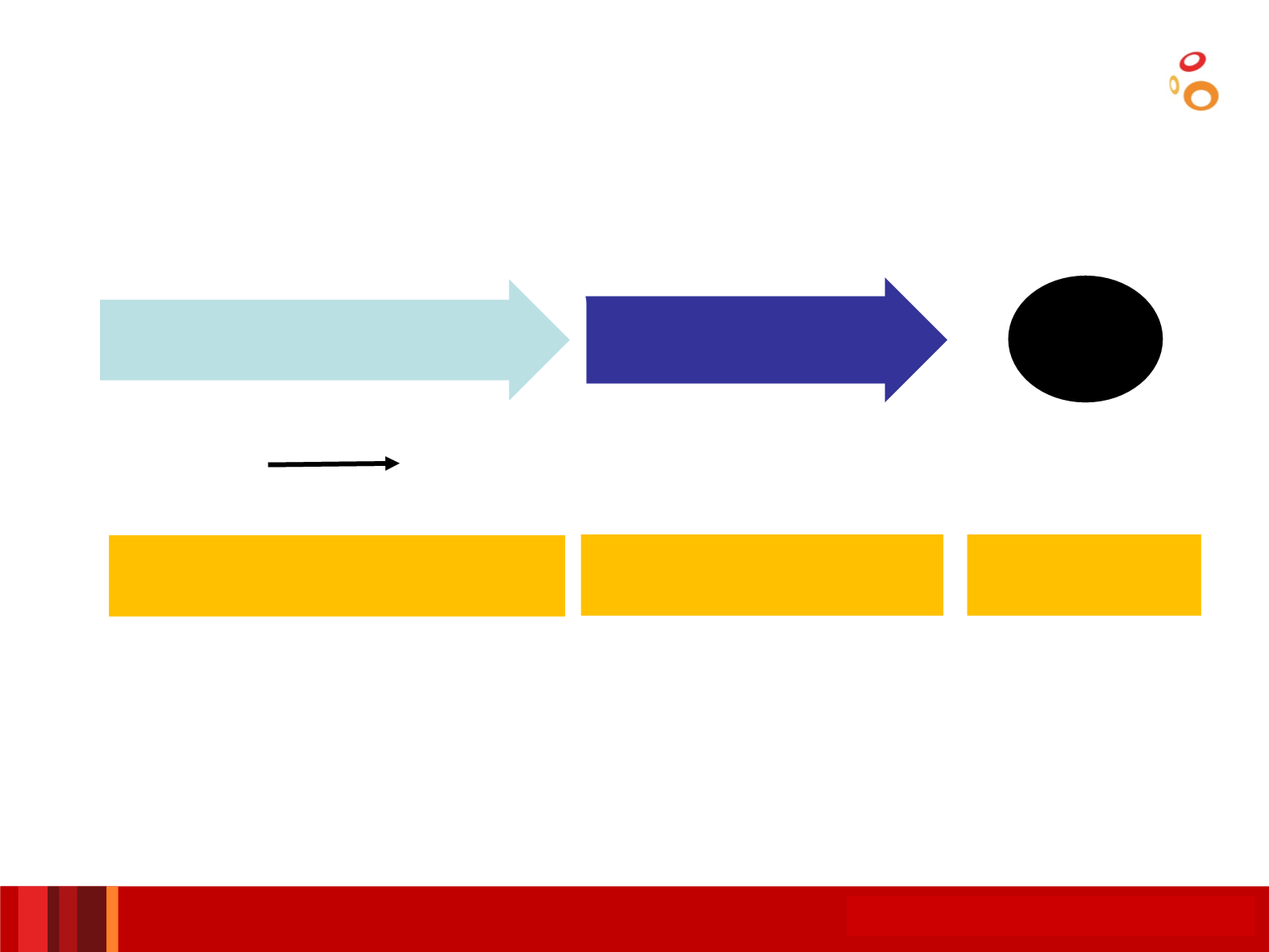
Standard of care in R/M disease
•
Taxane ± platin
•
Cetuximab* ± taxane
•
Cetuximab*
•
Methotrexate
<10%
•
2nd line
•NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers. V.1.2015.
•SEOM Clinical Guidelines in HNC. CTO v.2017
80%
•
Cetuximab
+ platinum-based CT
•
Erbitax (No P
candidates; PS 2)
Cetuximab
maintenance
until PD
1st line
RR: 35-40%
Median SV: 10 mo
RR: <10%
Median SV: 3-5 mo
CT, chemotherapy; Mo, months; PS, performance status;
R/M, recurrent or metastatic; RR, recurrence rate; SV, survival.
3rd line
•
Individualise
(slow PROG //
previous RR)
•
Methotrexate
RR: Anecdotic
Median SV: ??









