
Inst itut Català d'Oncologia
Institut Català d’Oncol gia
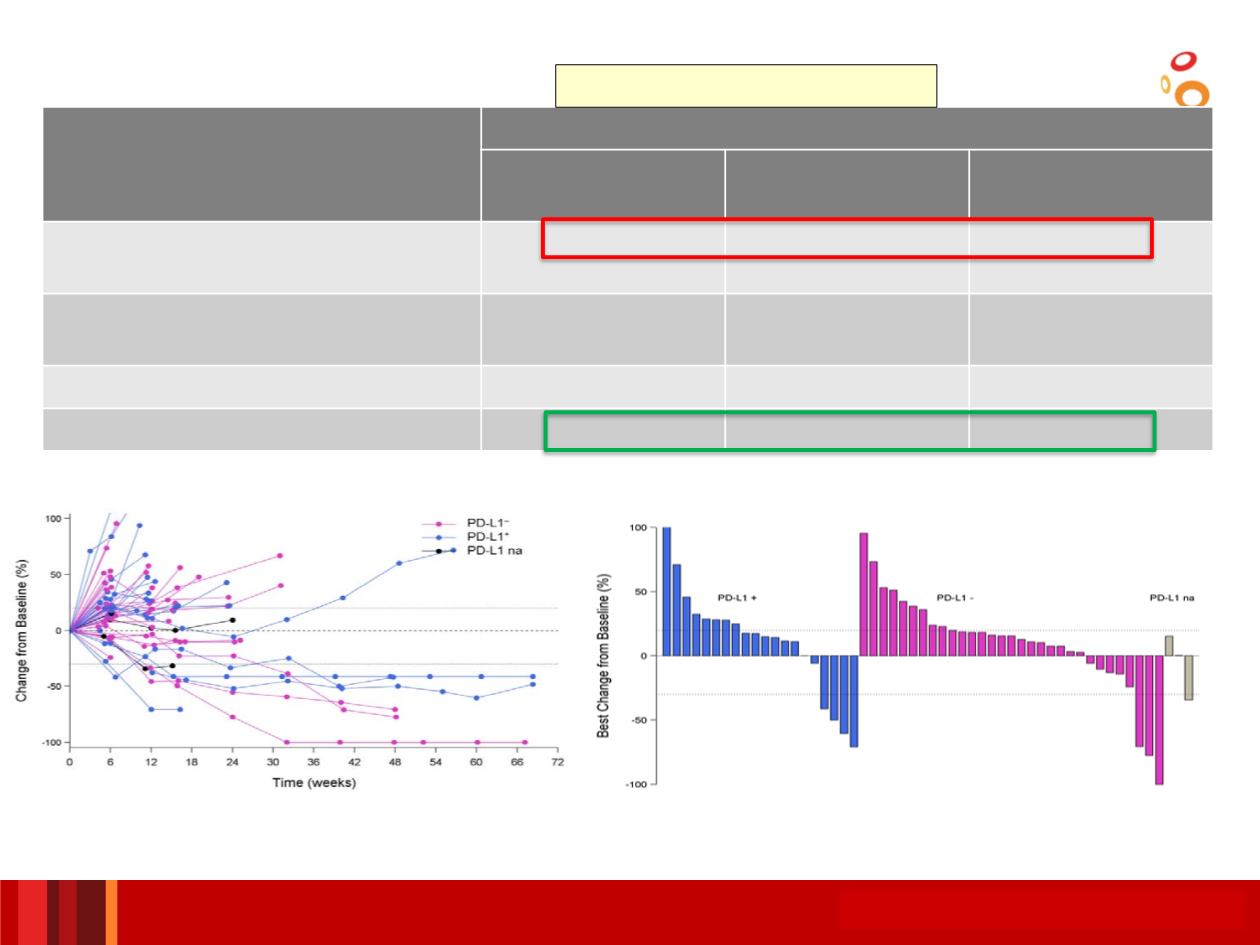
SCCHN Tumor Response Overall and by PD-L1 Status
Durvalumab Phase I
12
PD-L1 status was determined via the PD-L1 (SP263) immunohistochemical assay
Durvalumab 10 mg/kg
All patients
(n=62)
PD-L1
+
(n=22)
PD-L1
–
(n=37)
RECIST response (ORR), n/N (%)
95% CI
7/62 (11)
4.7–21.9
4/22 (18)
5.2–40.3
3/37 (8)
1.7–21.9
DCR 24 weeks
a
, n/N (%)
95% CI
9/62 (15)
6.9–25.8
4/22 (18)
5.2–40.3
4/37 (11)
3.0–25.4
Range of ongoing DoR
b
, weeks
16.1+–55.4+
41.1+–53.1+
16.1+–55.4+
Ongoing responders, n/N (%)
5/7 (71)
2/4 (50)
3/3 (100)
•
Tumor shrinkage by PD-L1 status (n=54)
•
Best change in tumor size from baseline by PD-L1 status (n=54)
Tumor PD-L1. VENTANA SP263









