
Inst itut Català d'Oncologia
Institut Català d’Oncol gia
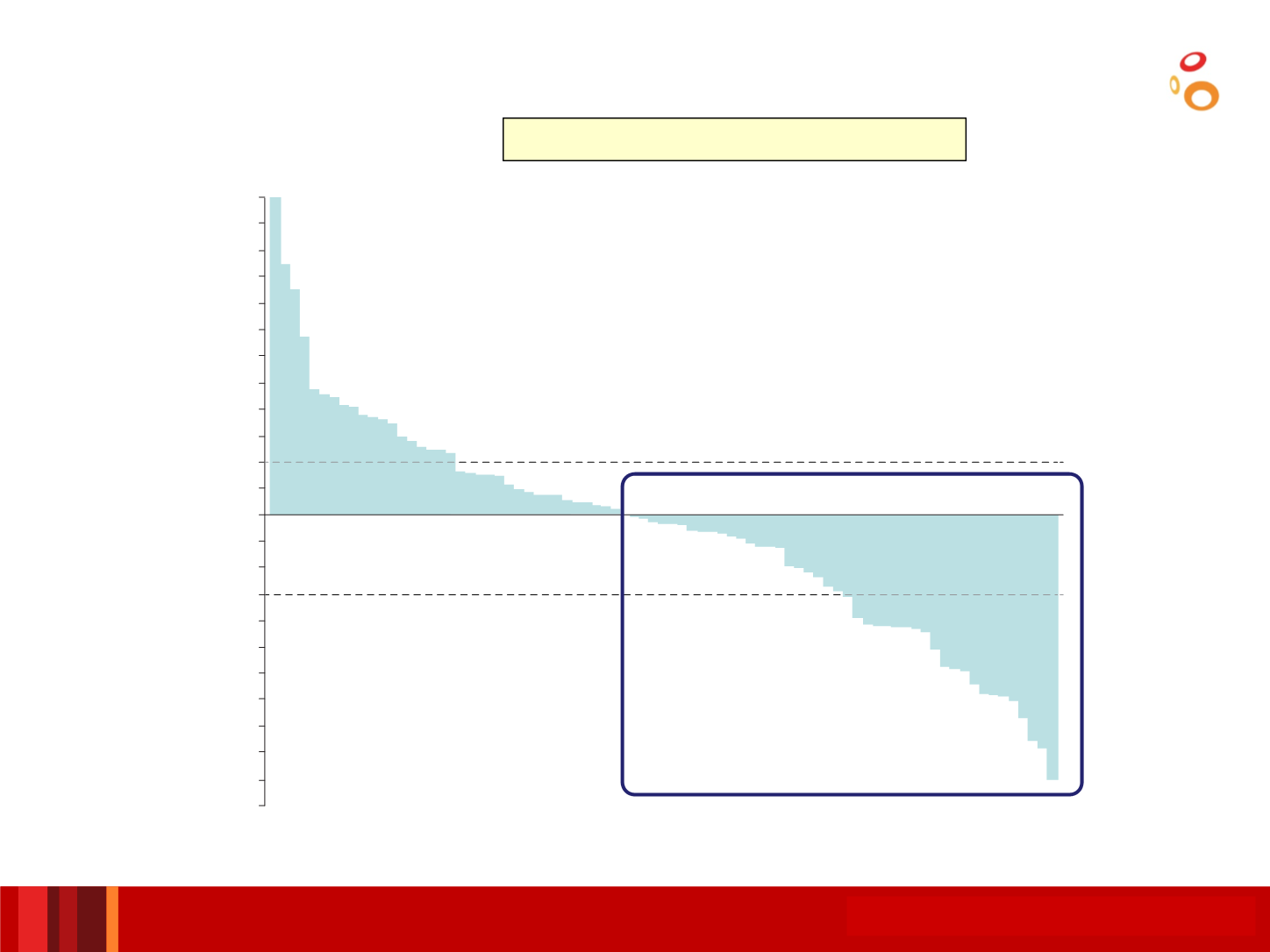
*Full analysis set, N=112; BICR assessment using RECIST v1.1.
Best % change from baseline in tumour size*
Durvalumab (N=112)
-110
-100
-90
-80
-70
-60
-50
-40
-30
-20
-10
0
10
20
30
40
50
60
70
80
90
100
110
120
•Best change from baseline in target lesion size (%)
•
40% of patients had a decrease
in
size of
target lesions
BICR, blinded independent central review; RECIST,
Response Evaluation Criteria In Solid Tumors.
HAWK:
single arm phase 2 trial in recurrent/metastatic HNSCC
Tumor PD-L1≥ 25% - VENTANA SP263
Zambert DP. ESMO 2017









