
Peeters M, et al. Eur J Cancer 2013;49(Suppl 4):abstract MC13-0024 (and poster).
†
Disease progression or intolerability.
Iri, irinotecan; Ox, oxaliplatin;
Q2W, every 2 weeks; Q8W, every 8 weeks.
Study endpoints: PFS (1
°
); OS, ORR, safety
mCRC
1
(n = 1183)
1:1
Disease assessment Q8W
FOLFOX4
(Q2W)
+ panitumumab
6 mg/kg
(Q2W)
FOLFOX4
(Q2W)
E
n
d
o
f
t
r
e
a
t
m
e
n
t
†
+ anti-VEGF
(n = 55)
‒ anti-VEGF
(n = 114)
+ anti-VEGF
(n = 45)
‒ anti-VEGF
(n = 132)
R
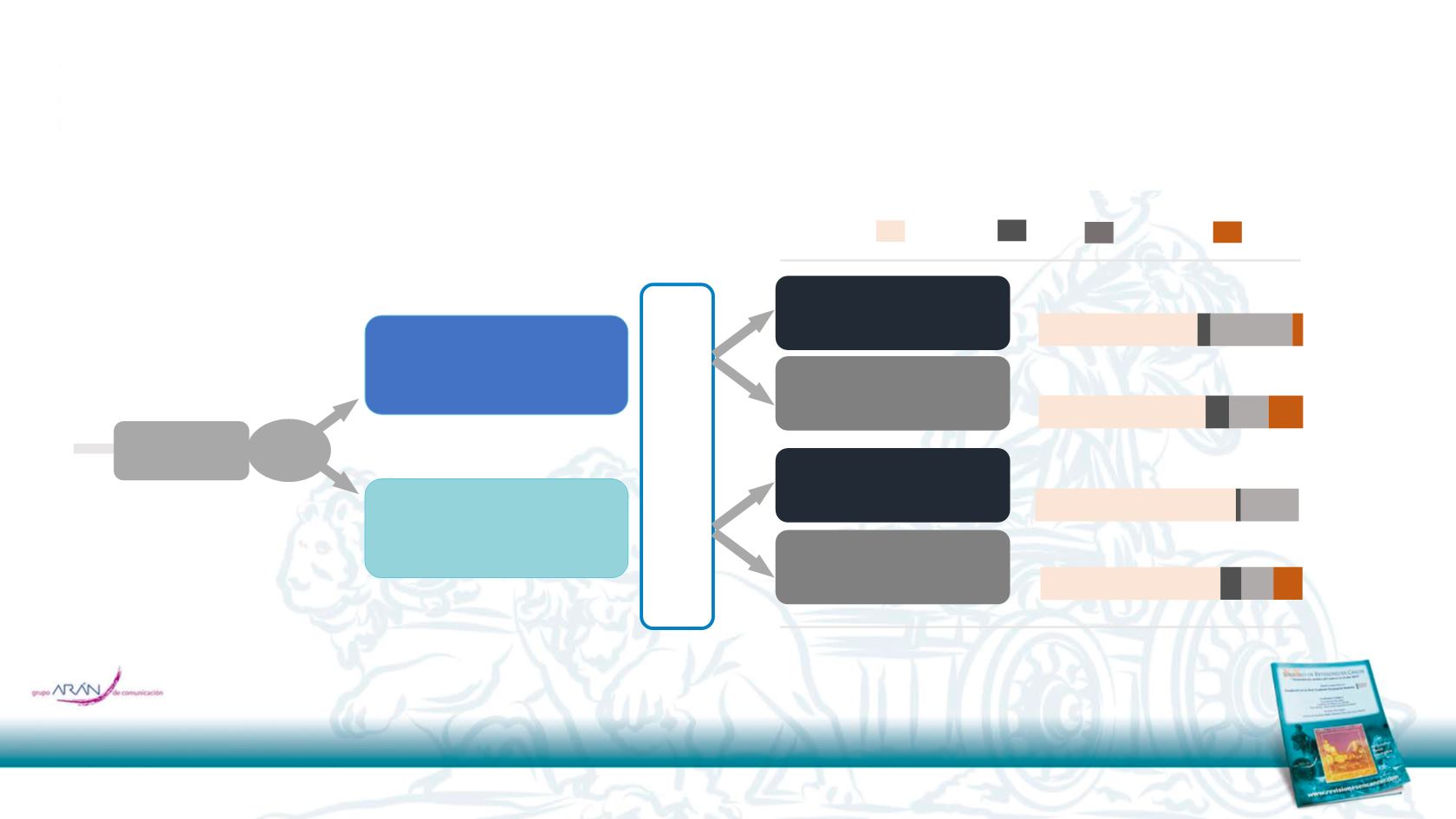
Iri-based Ox-based Both Unknown
60 5 31 4
63 9 15 13
76 2 22
68 8 12 11
%
%
%
%
PRIME Post-progression anti-VEGF therapy
(post-hoc analysis)
Post-protocol treatment WT
RAS
population









