
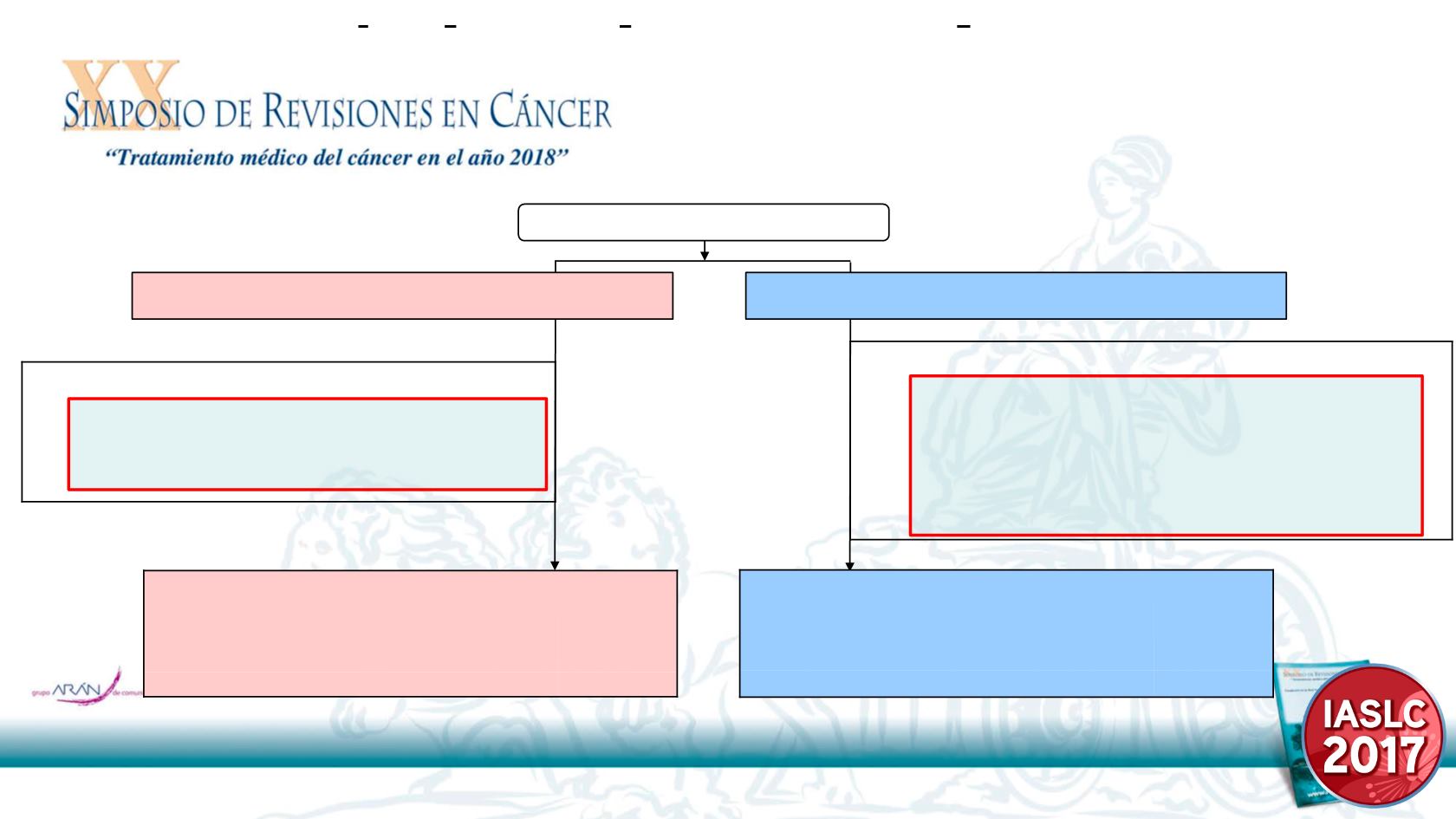
Enrollment period:
Sep. 2012~May. 2015
All efficacy analyses were conducted on the ITT population (all randomized patients).
ITT, intend to treat, Tx, treatment, PD, disease progression
* No study medication received
Enrolled n=102
Abstract ID: 8717
•
No study medication received
n=1
•
Major Protocol Violation
n=4
– No R0 resection
n=3
– Critical assessment deficiency
n=1
•
No study medication received
n=8
•
Major Protocol Violation
n=11
– No R0 resection
n=5
– Critical assessment deficiency
n=3
– Prior anti-neoplastic Tx
n=1
– Metastatic before study Tx *
n=1
– Other anti-neoplastic Tx before PD
n=1
Randomized to Erlotinib n=51
Randomized to Chemotherapy n=51
Available for primary analysis
•
ITT analysis population
n=51
•
Safety analysis population
n=50
•
Per-protocol population
n=46
Available for primary analysis
•
ITT analysis population
n=51
•
Safety analysis population
n=43
•
Per-protocol population
n=33
Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage IIIA
EGFR
Mutant NSCLC Patients (EVAN, NCT01683175)









