
Cancer (NSCLC) with
EGFR
-activating Mutation (ADJUVANT): A Randomized, Phase III Trial (CTONG 1104)
•
Key results
•
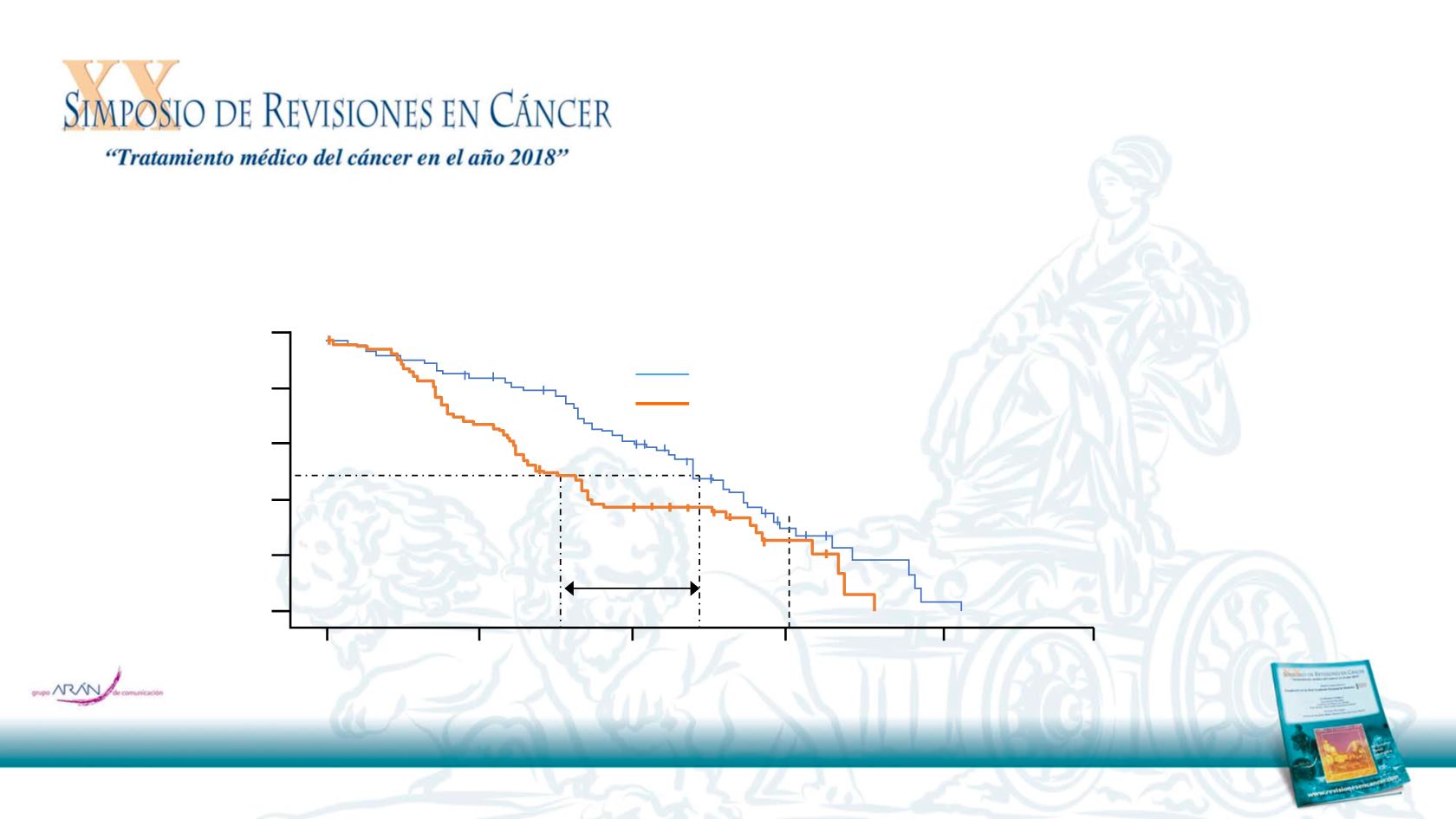
Approximately 65% of patients were stage IIIA, 30% were stage IIA and 4% stage IIB
•
Gefitinib was associated with longer DFS than vinorelbine + cisplatin
•
3-year DFS rate was 34% with gefitinib vs. 27% with vinorelbine + cisplatin
Wu L-Y et al. J Clin Oncol 2017;35(suppl):Abstr 8500
Disease survival, %
Time, months
100
80
60
40
20
0
0
111
111
12
88
54
24
57
26
36
10
5
48
1
0
60
0
No. at risk:
Gefitinib
Vinorelbine + cisplatin
Group
n
Events
Median, months (95%CI)
Gefitinib
111
65
28.7 (24.9, 32.5)
Vinorelbine + cisplatin
111
59
18.0 (13.6, 22.3)
HR for recurrence 0.60
95%CI 0.42, 0.87; p=0.005
Δ 10.7 mo
DFS









