
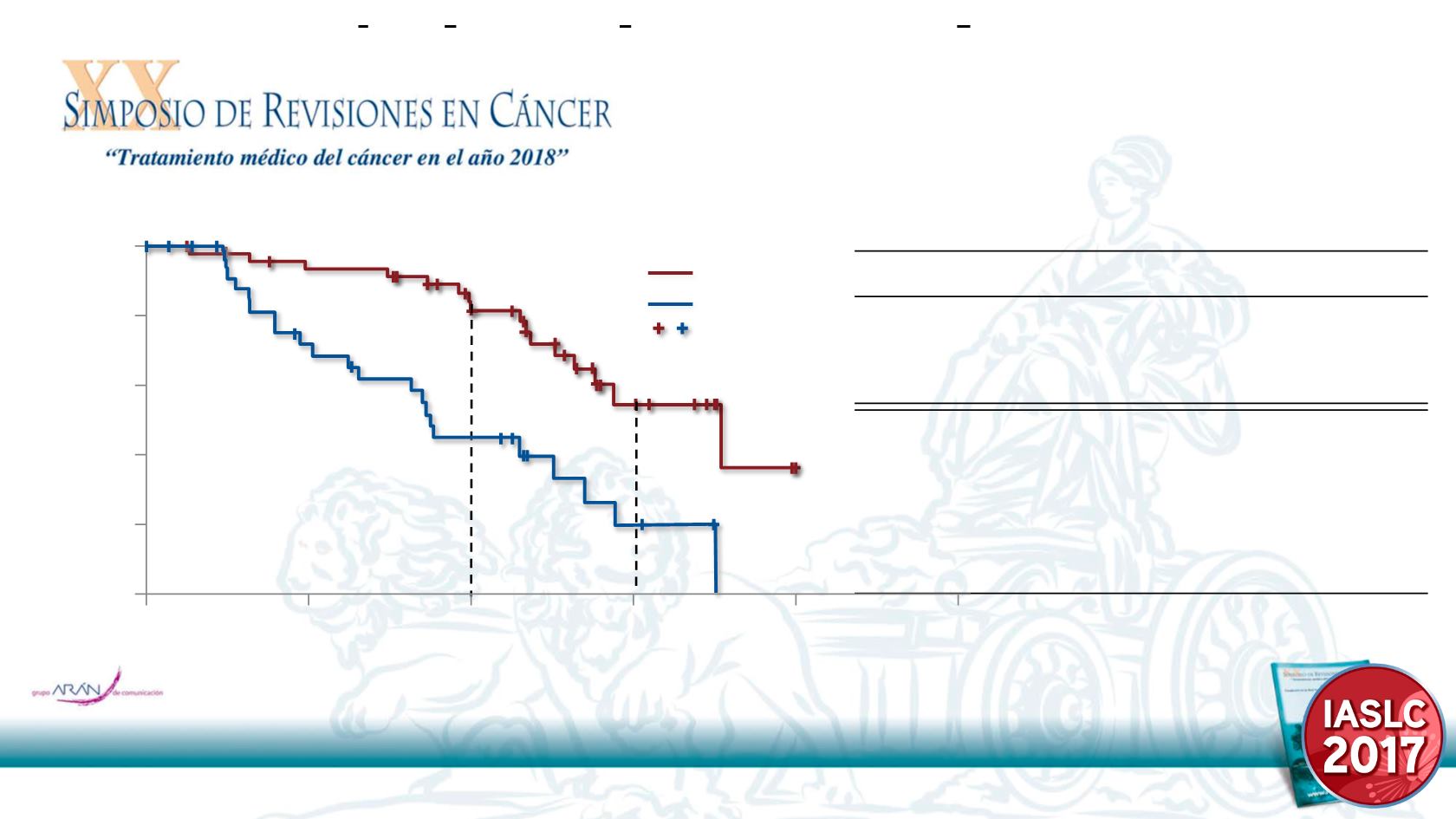
DFS (ITT Population)
Abstract ID: 8717
Patients with events, n (%)
16 (31.4)
22 (43.1)
Median DFS, months
(95% CI)
42.41
( 31.67, -)
20.96
( 12.29, 32.36)
HR (95% CI)
0.268 ( 0.136, 0.531)
P-value (log-rank test)
<0.001
P-value (stratified log-rank test)
<0.001
Data cut-off date: 2017-Jun-15, Median follow-up time was 33.2 months for Erlotinib and 28.1 months for NP
0
12
24
36
48
60
Time (Months)
0.0
0.2
0.4
0.6
0.8
1.0
DFS probability
Patients at Risk
Erlotinib
NP
51
51
42
22
29
13
9
3
0
0
NP
Censored
Erlotinib
81.35%
44.62%
Erlotinib
(n=51)
NP
(n=51)
2-year DFSR, %
81.35
44.62
P-value
<0.001
3-year DFSR, %
54.24
19.83
P-value
0.011
54.24%
19.83%
Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage IIIA
EGFR
Mutant NSCLC Patients (EVAN, NCT01683175)









