
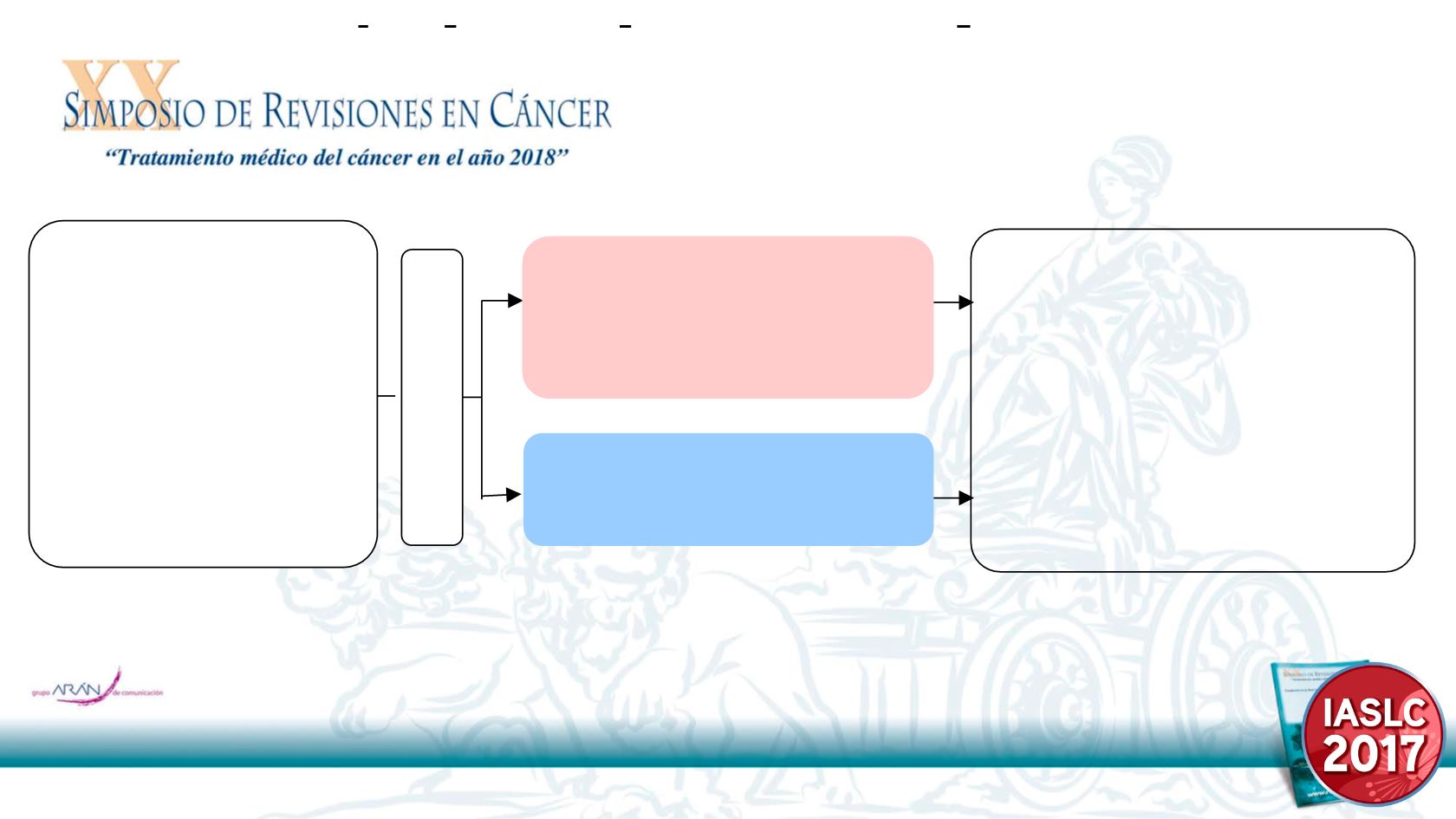
EVAN Study Design
Stratification factors:
•
EGFR mutation type: Exon 19 vs. 21
•
Histology: Adenocarcinoma vs. Non-adenocarcinoma
•
Smoking status: Smoker vs. Non-smoker*
NCT01683175
NSCLC, non-small-cell lung cancer; EGFR, Epidermal growth factor receptor; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PO, by mouth; QD, once daily; i.v. intravenous drip; DFS, disease-free survival; OS, overall survival;
NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; QoL, quality of life
Abstract ID: 8717
# 7th Edition of the TNM classification
& IASLC definition, Lung Cancer 2005; 49:25-33
* Non-smoker was defined as patient who never smoke or had smoked
≤
100 cigarettes in the lifetime, all the others defined as smoker
Vinorelbine 25mg/m
2
i.v. d1,8,
Cisplatin 75mg/m
2
i.v. d1,
21d
×
4 cycles
Erlotinib
150mg PO QD for 2 years,
or until relapse
or unacceptable toxicity
Randomization 1:1
KEY ELIGIBILITY
•
Stage IIIA NSCLC
#
•
R0 resection
&
•
No previous anti-tumor treatment
•
EGFR
Exon 19 or 21 mutation
•
Age
≧
18 years &
≦
75 years
•
ECOG PS 0–1
ENDPOINTS
•
Primary endpoint:
–
2-year DFSR
•
Secondary endpoints:
–
DFS
–
OS
–
Safety (NCI CTCAE 4.0)
–
QoL
–
Exploratory biomarker analysis
N=94
Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage IIIA
EGFR
Mutant NSCLC Patients (EVAN, NCT01683175)









