
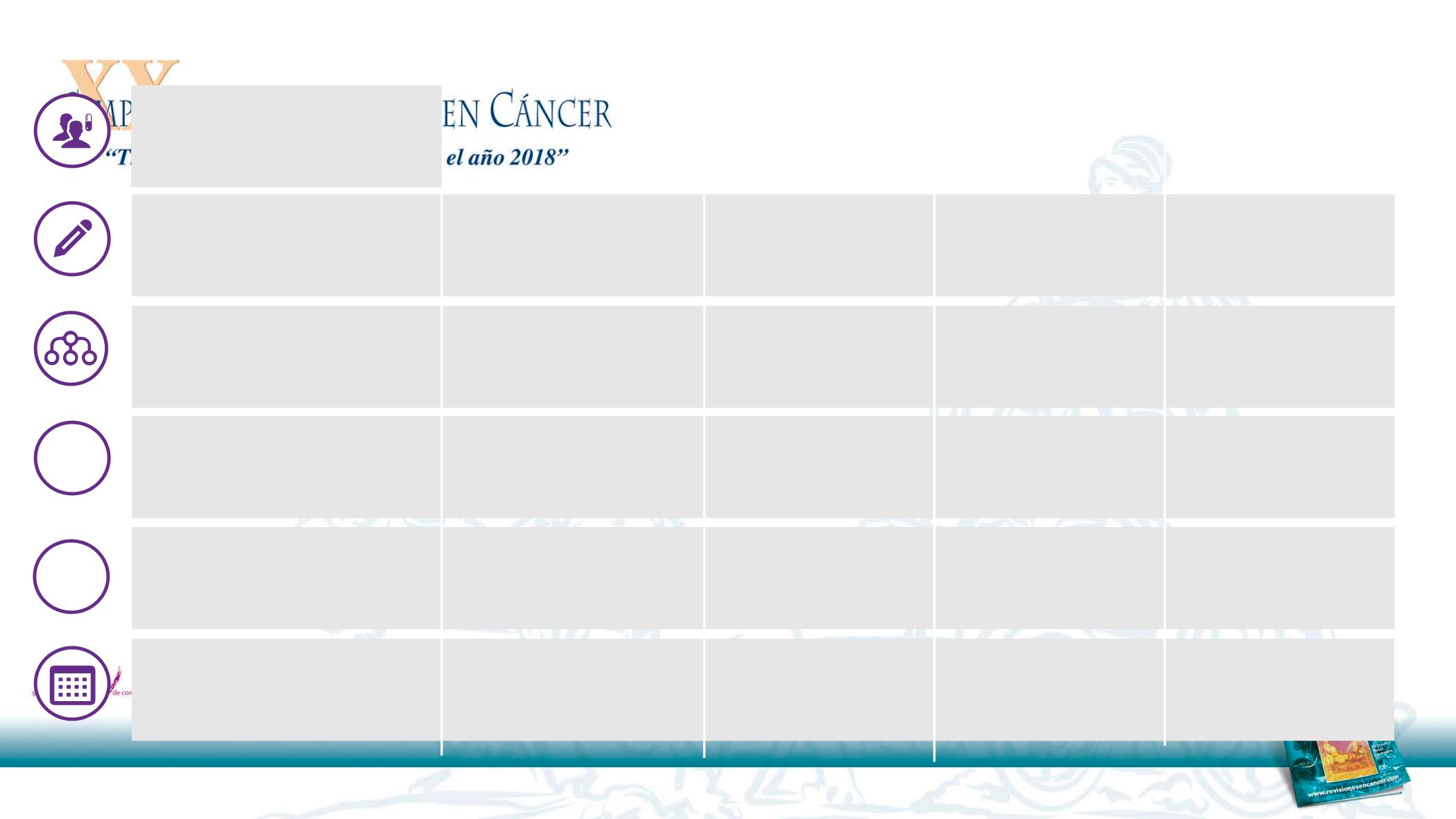
Study
Design
Recruiting
Secondary
Endpoints
Study
Period
Primary
Endpoint
1
2
ACCRU RC1126 (NCT01532089)
USA
•
Phases III
•
Erlotinib+Bec vs
Erlotinib
•
EGFR Mut (+)
Recruiting
PFS
OS
ORR
Safety
2012–2017
BEVERLY
(NCT02633189)
Italy
2015 – 2018
OS
QoL
ORR
•
Phases III
•
Erlotinib+Bec vs
Erlotinib
•
EGFR Mut (+)
NEJ026
(UMIN000017069)
Japan
20
15–2018
•
Phases III
•
Erlotinib+Bec vs
Erlotinib
•
EGFR Mut (+)
ARTEMIS
(NCT02759614)
China
2016–2019
•
Phases III
•
Erlotinib+Bec vs
Erlotinib
•
EGFR Mut (+)
Recruiting
Recruiting
Status
Study
No.
PFS
PFS
PFS
OS
ORR
Safety
OS
QoL
ORR
Ongoing Study: erlotinib + bevacizumab
vs.
erlotinib as first-line treatment









