
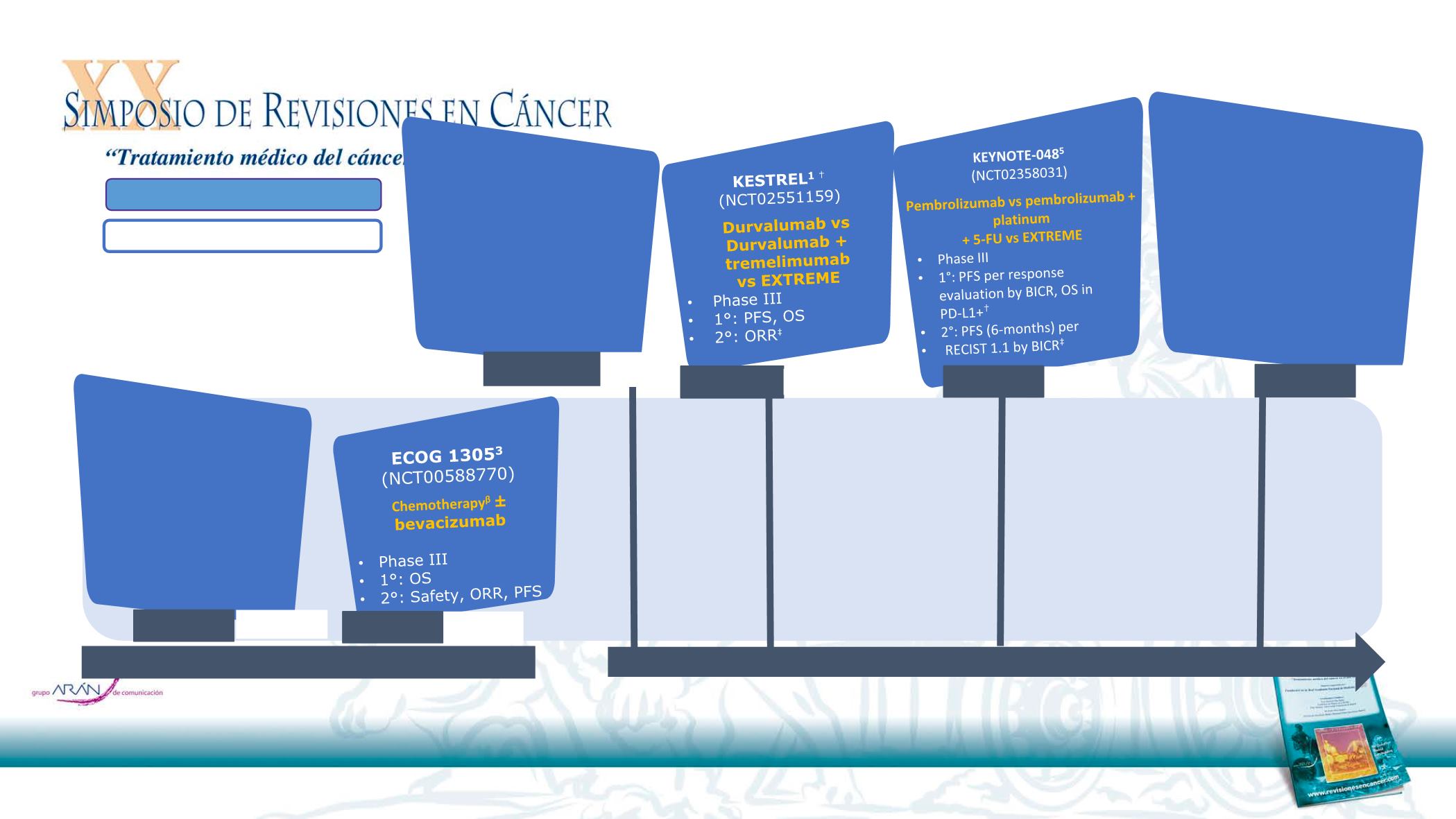
CheckMate 651
6
(NCT02741570)
Nivolumab + ipilimumab
vs EXTREME
•
Phase III
•
1L (≥6 months after CT as part of
multimodal treatment)
•
1°: OS, PFS (both 3 year)
•
2°: ORR (3 year), TTD
IO
Traditional
2018
2020
2017
2018/2019
39
1ªL CCC: Estudios en marcha
•
1.
https://clinicaltrials.gov/ct2/show/NCT022686952.
https://clinicaltrials.gov/ct2/show/NCT00588770•
3.
https://clinicaltrials.gov/ct2/show/NCT028235744.
https://clinicaltrials.gov/ct2/show/NCT02551159•
5.
https://clinicaltrials.gov/ct2/show/NCT023580316.
https://clinicaltrials.gov/ct2/show/NCT02741570*All studies were randomized and open-label, except for CheckMate 714, which was double-blind;
†
Study stopped for safety reasons
‡ Other endpoints not listed;
‡
Patients received docetaxel + cisplatin, docetaxel + carboplatin, cisplatin + 5-FU or carboplatin +5-FU
BIRC, blinded independent central review; QoL, quality of life; ORR, objective response rate; OS, overall survival; PFS, progression-free
survival; RECIST, Response Evaluation Criteria in Solid Tumors Version 1.1; TTD, time to deterioration
Primary completion date
Estimated enrollment
CheckMate 714
3
(NCT02823574)
Nivolumab vs
Nivolumab + ipilimumab
•
Phase II
•
1°: ORR (19 months) in
platinum-refractory
•
2°: ORR in platinum-
eligible, PFS, OS (all 19
months)
N=315
02/2018
N=490
01/2019
TPExtreme
2
(NCT02268695)
EXTREME vs
Cisplatin + docetaxel
+ cetuximab
•
Phase II
•
1°: OS
•
2°: RR, PFS, TTP,
safety,
QoL, compliance, cost
12/2017
N=416
N=400
12/2017
03/2018
N=760
N=825
03/2018









