
34
•
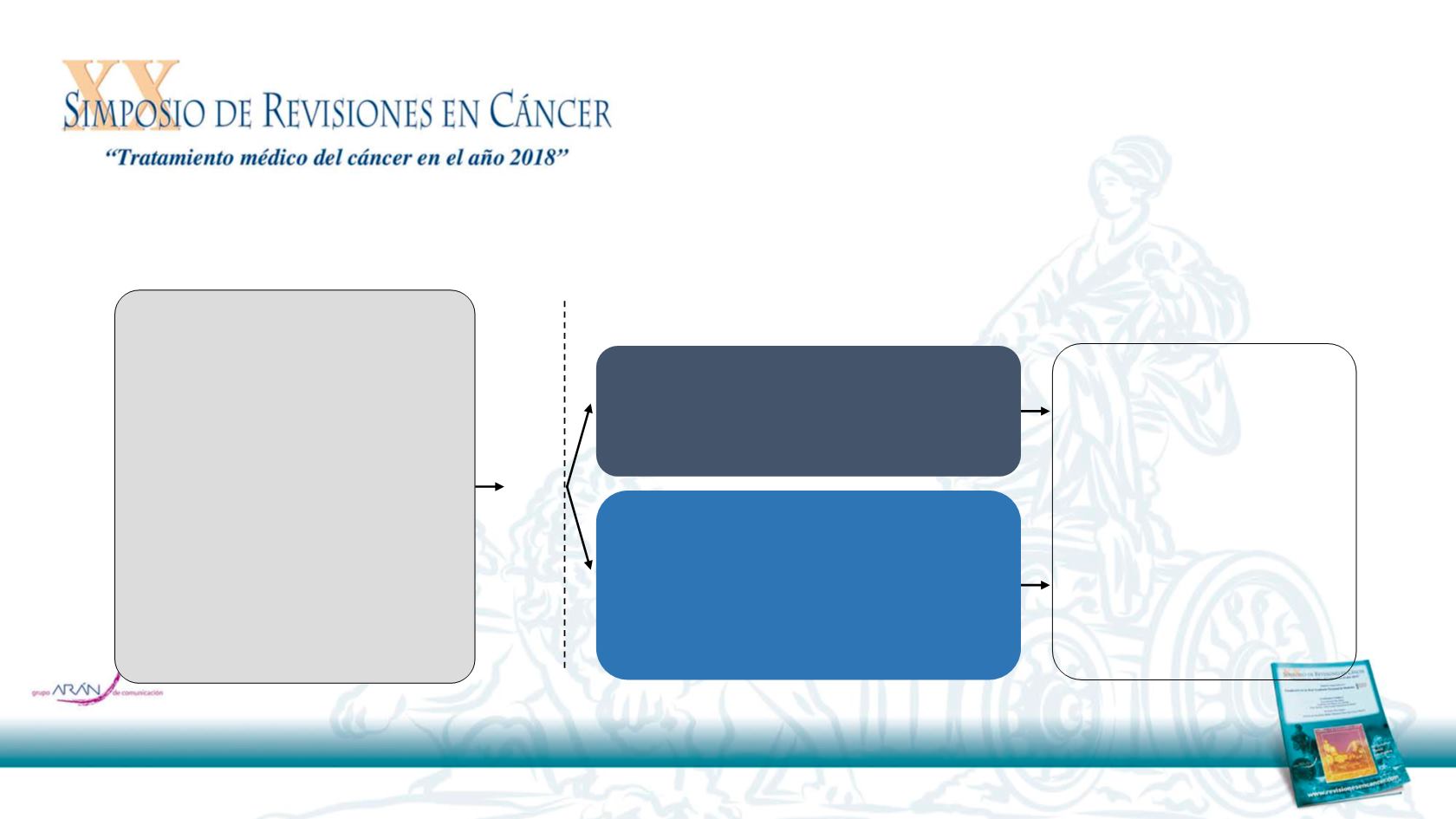
CheckMate 141 was a phase 3, randomized, open-label trial in patients with platinum-refractory R/M
SCCHN
DOR = duration of response; HPV = human papillomavirus; IV = intravenous; PD-L1 = programmed death ligand 1; PFS = progression-free survival; QoL = quality of life
Primary endpoint
•
OS
Other endpoints
•
PFS
•
ORR
•
DOR
•
Safety
•
Biomarkers
•
Patient-reported QoL
Key eligibility criteria
•
R/M SCCHN of the oral cavity,
pharynx, or larynx
•
Progression on or within
6 months of last dose of
platinum-based therapy
•
Irrespective of number of prior
lines of therapy
•
Documentation of p16 to
determine HPV status
(oropharyngeal cancer only)
•
Regardless of PD-L1 status
Nivolumab
3 mg/kg IV every 2 weeks
(n = 240)
Investigator’s choice
•
Methotrexate 40 mg/m
2
IV weekly
•
Docetaxel 30 mg/m
2
IV weekly
•
Cetuximab 400 mg/m
2
IV once, then
250 mg/m
2
weekly
(n = 121)
Randomized 2:1
(stratified by prior cetuximab)









