
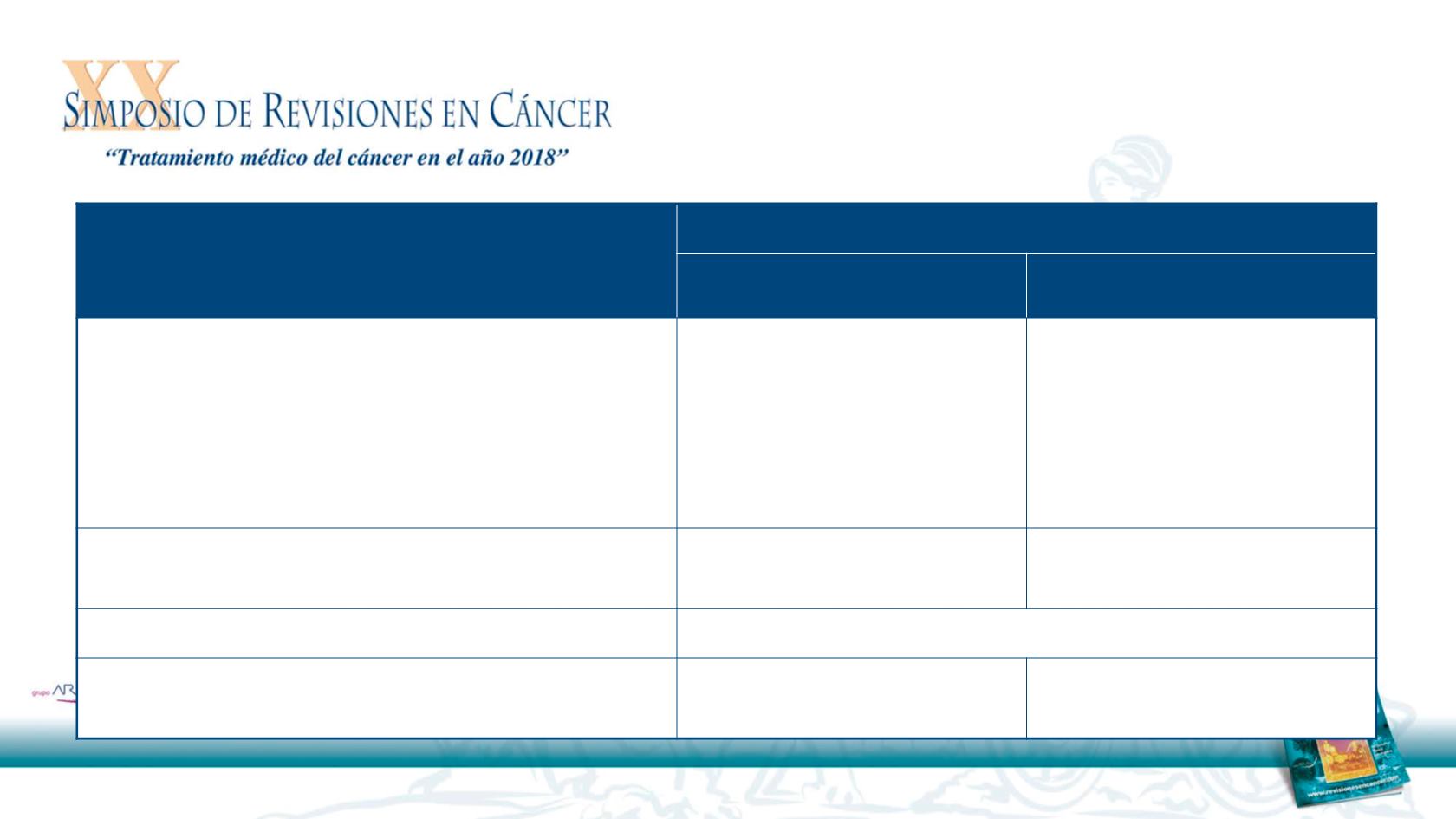
37
Received study drug in 1L R/M setting
Nivolumab
(n = 52)
Investigator’s choice
(n = 26)
Best overall response, n (%)
Complete response
Partial response
Stable disease
Progressive disease
Unable to determine
2 (3.8)
8 (15.4)
11 (21.2)
18 (34.6)
13 (25.0)
0
3 (11.5)
8 (30.8)
8 (30.8)
7 (26.9)
ORR, n (%)
[95% CI]
10 (19.2)
[9.6, 32.5]
3 (11.5)
[2.4, 30.2]
Odds ratio (95% CI)
1.83 (0.46, 7.31)
Time to response, median (range), months
2.0 (1.8
–
6.3)
2.0 (1.9
–
4.6)
RR 1L R/M nivolumab or IC after platinum-based
therapy in the primary/adjuvant setting









