
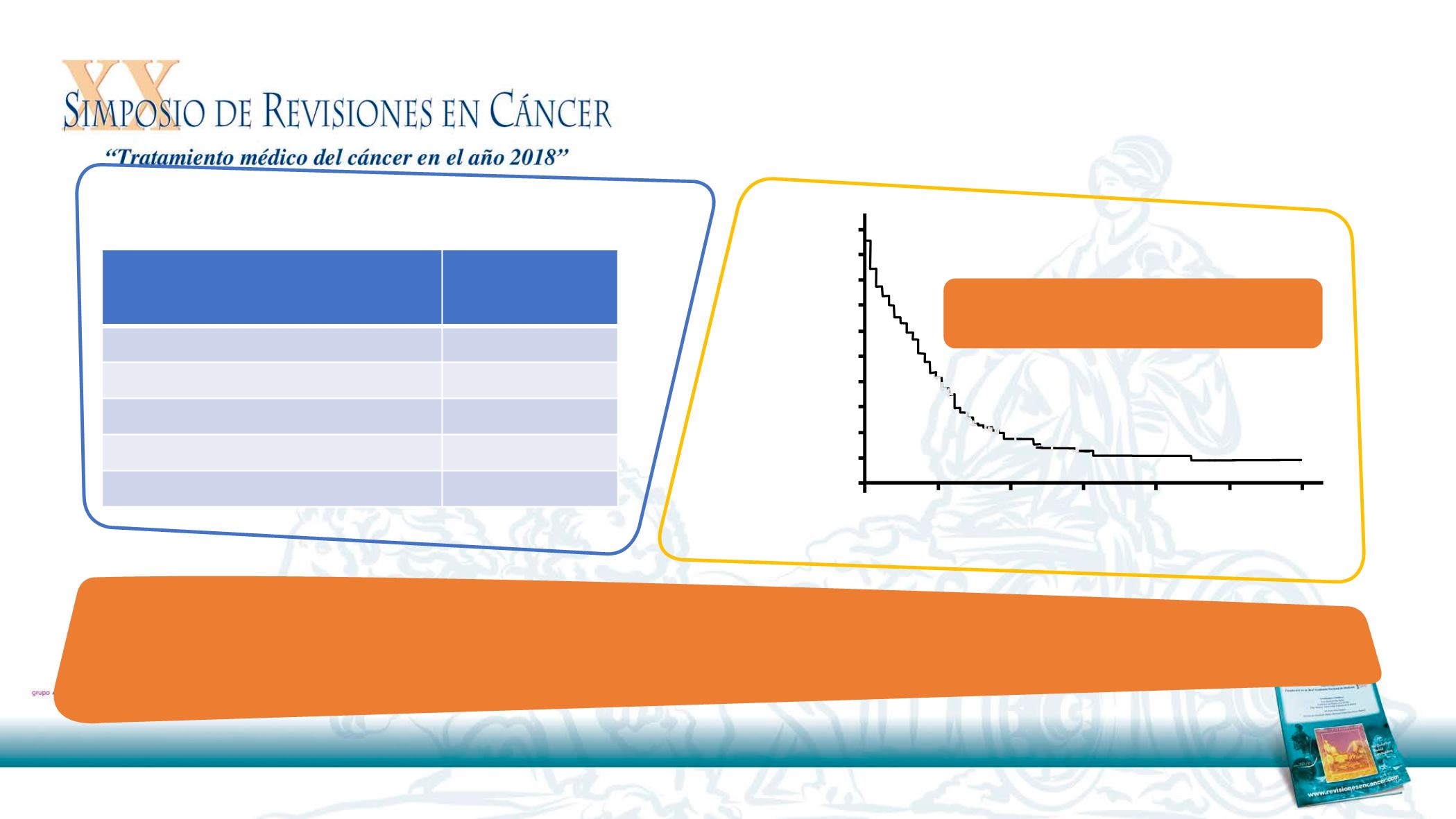
Author’s conclusion:
Paclitaxel + cetuximab showed similar OS & PFS in unselected patients with R/M SCCHN as previously reported in a Phase II
trial, confirming its value in unfit patients
1,2
32
•
1. Pajares Bernad I, et al. ESMO 2016 (Abstract 991P)
•
2. Hitt R, et al. Ann Oncol 2012;23:1016–22
Image courtesy of AlfredoCreates.com via the Noun Project
•
*QW paclitaxel + cetuximab is an off-label treatment in R/M SCCHN
100
90
80
70
60
50
40
30
20
10
0
0
12
24
38
48
60
72
OS (%)
Time (months)
Median OS, 10 months
(95% CI 8.3–11.7)
1
Efficacy
1
Cetuximab +
paclitaxel*
(N=148)
ORR, %
47.3
SD, %
20.3
PD,%
32.4
Median OS, months (95% CI)
10 (8.3–11.7)
Median PFS, months (95% CI)
7 (5.9–8.1)









