
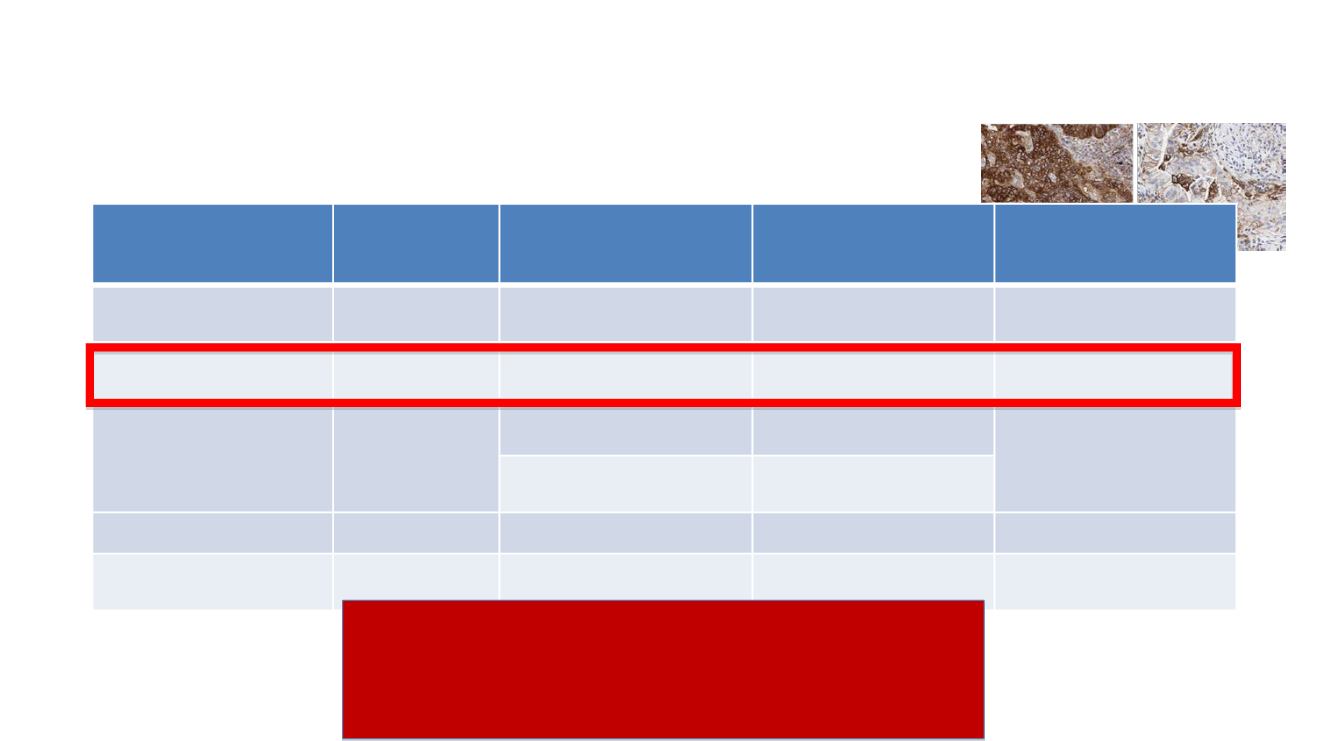
PD-L1 immunohistochemistry in NSCLC
Tsao -WCLC 2017 (Courtesy of Prof. Besse)
Drug
PD-L1 IHC
Assay
PD-L1 scoring
Cut-offs reported
in clinical trials
FDA Diagnostic
Status
Nivolumab
28-8
Tumor cells
1%, 5%, 10%
Complementary
Pembrolizumab
22C3
Tumor cells (TPS)
1%, 50%
Companion
Atezolizumab
SP142
Tumor cells (TC)
1%, 5%, 50%
Complementary
Immune cells (IC)
1%, 5%, 10%
Durvalumab
SP263
Tumor cells
25%
Unknown
Avelumab
73-10
Tumor cells
1%, 50%, 80%
Unknown
Companion diagnosis: REQUIRED for the safe and effective use of a drug
Complementary diagnostic: NOT REQUIRED but can provide additional information
TPS: tumor proportional score; TC: staining on tumor cell; IC: staining on immune cells
US trusts in Companion Dx
EU trusts in their pathologists!









