
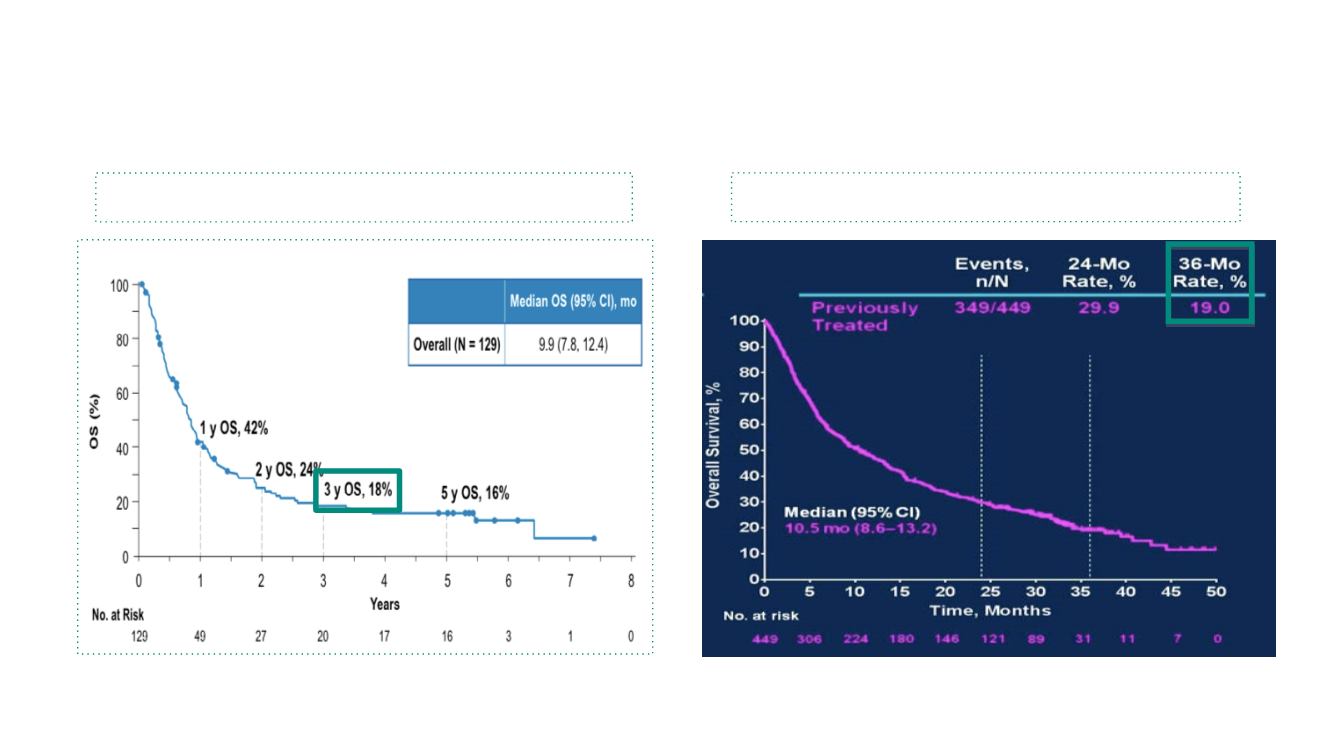
The tail effect with immunotherapy
8
th
TNM IASLC Classification, 3-y OS M1c: ~8%
5y follow up phase I CA209-003 Trial
PD-L1 > 50% (N=13)
5-yOS: 43%
Nivolumab up to 96 weeks
PD-L1 > 50% (N=138)
3yOS: 29.7%
Pembrolizumab up to PD
3-y OS phase I KEYNOTE 001 Trial
Brahmer – AACR 2017 * Vokes – Ann Oncol 2018 * Eberhardt – J Thorac Oncol 2015 * Leighl– ASCO 2017









